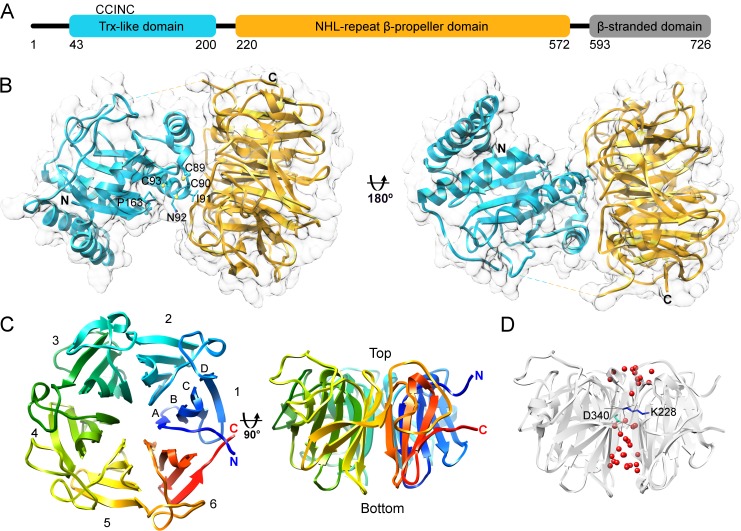
Fig 1. Human NHLRC2 structure.
(A) Schematic representation of the domain architecture of human NHLRC2. (B) Ribbon and surface representation of NHLRC2 (9–572) crystal structure with the Trx-like domain colored in cyan and the β-propeller colored in gold. Residues of CCINC motif are shown. Cysteine residues are shown in ball and stick representation and neighboring residues are shown in stick representation. The missing loop connecting the two domains is shown with a dashed line. (C) Two views of NHLRC2 β-propeller are related by 90° rotation around x-axis. Blades are indicated by numbers and strand location is indicated by capital letters. (D) The solvent channel inside of the β-propeller domain contains water molecules (red) and is disrupted by Lys-228 (blue) and Asp-340 (cyan) forming hydrogen bonds with waters and backbone groups of adjacent blades.