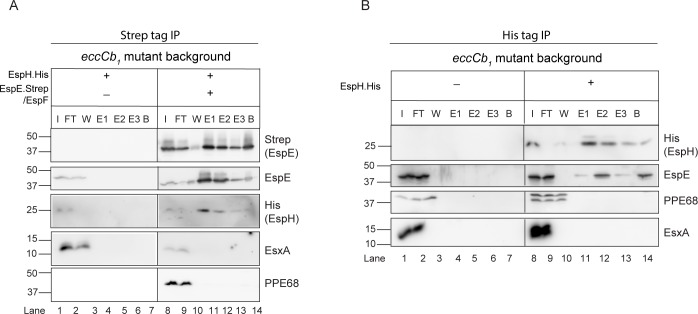
Fig 5. EspH specifically interacts with EspE in M. marinum.
A. Immunoblots of pulldown assays using Strep-tactin agarose. EspE-Strep was purified from soluble lysates of the eccCb1 mutant expressing only EspE-Strep/EspF or EspE-Strep/EspF together with EspH-His. A strain containing empty plasmids was included as negative control. Total input material (I), unbound proteins (FT), the final washing step (W), three fractions of eluted proteins (E1, E2, E3) and boiled beads fractions were separated by SDS-PAGE and further immunoblotted using antisera directed against the Strep- or His-tag. The elution fractions were loaded 10 times more compared to the other fractions. Endogenous EspE, PPE68 and EsxA substrates were detected using anti-EspE, anti-PPE68 and anti-EsxA, respectively. B. Immunoblots of pulldown assays using Ni-NTA beads. EspH-His proteins were purified from soluble lysates of the eccCb1 strain carrying a plasmid expressing EspH-His or the corresponding empty plasmid. Total input material (I), unbound proteins (FT), the last washing step (W), proteins eluted with 50 mM (E1), 100 mM (E2), and 200 mM (E3) imidazole and boiled bead fraction were separated by SDS-PAGE and probed with His-specific antiserum. The elution fractions were loaded 10 times more compared to the other fractions. Endogenous EspE, PPE68 and EsxA proteins were detected using anti-EspE, anti-PPE68 and anti-EsxA, respectively.