Abstract
We report results on PCDD/F formation over iron (III) oxides catalysts for a mixture of 2-monochlorophenol (2-MCP) and 1,2-dichlorobenzene (1,2-DCBz) for both oxidation and pyrolysis. Competitive adsorption between chlorinated benzenes and chlorinated phenols affects the transformation of these precursors and plays a crucial role in the PCDD/F formation in mixed MCP/1,2-DCBz-feed streams. Comparing the integrated PCDD and PCDF yields, it becomes apparent that with decreasing 2-MCP content in the feed stream the PCDF yield first rises and then levels off, at ~0.4% for pyrolytic and at ~0.6% for oxidative conditions. Present results further confirm that the PCDD/PCDF-ratio cannot be used to validate the de novo pathway nor can it be used as an indicator of de novo synthesis in incinerators. In fact, the PCDD/PCDF-ratio is strongly dependent on the relative concentration of these two precursors in the reacting stream, i.e., chlorinated benzenes vs. chlorinated phenols.
Keywords: PCDD/Fs, iron (III) oxide, precursor pathway, de novo, dioxin formation
1 Introduction
Emission of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/F) from thermal and combustion processes constitutes a major toxic component of environmental pollutants (Environmental Protection Agency, 2001; Brzuzy and Hites, 1996; Fiedler, 1998; Thomas and Spiro, 1996). It has been well established that transition metal-mediated reactions account for the majority of PCDD/F formation in combustion systems (Huang and Buekens, 1995; Olie et al., 1998, Altwicker et al., 1990). In particular, probably both the copper and iron in combustion generated particulate matter (Cains et al., 1997) are active in catalysing PCDD/F formation from most combustion sources (Stieglitz, 1991; Ismo et al., 1997). Furthermore, it has been noted that typically transition metal-mediated reactions occur in the low temperature post-combustion zone and the air pollution control devices (Dickson et al., 1989; Ghorishi and Altwicker, 1995). The catalytic role of those metals is strongly associated with two different reaction schemes: the de novo formation pathway, or else precursor routes. There is still wide debate between scientists on which model better represents the observed PCDD/F emissions: the de novo or the precursor model. Notably, numerous studies have been reported in regard to surface-mediated processes of PCDD/F formation via de novo synthesis (Stieglitz et al., 1989; Wikström et al., 2003a; Stieglitz, 1998) and transition metal-mediated process from reactions of aromatic precursors (Addink and Olie, 1995; Milligan and Altwicker, 1996; Evans and Dellinger, 2005). Generally, propagated is the view that the PCDD to PCDF ratio indicates which mechanism is dominating these emissions, where PCDD/PCDF < 1 is supposedly an attribute of de novo synthesis (Xiao et al., 2014; Karademir and Korucu, 2013; Vermeulen et al., 2014). This stems from the fact that most experimental studies involving precursors perceived PCDD/PCDF-ratios bigger than one. However, recent studies have indicated that the type of the PCDD/F precursor and/or the speciation of the active metal are a critical factor in determining the PCDD/PCDF-ratio (Nganai et al., 2014, 2012, 2011). In fact, chlorinated benzenes (CBz) have been suggested to be precursors of (mostly) PCDF and the PCDD/PCDF-ratio observed in the exhaust of thermal sources is dependent on the relative concentration of chlorinated benzenes vs. chlorinated phenols (CPh) in the gas phase. Also, some researchers specified that the presence of other combustion by-products (present in large excess, compared to these two typical PCDD/F precursors) is critical in the formation of PCDD/F: by their in-situ transformation to such precursors they further increase the PCDD/F output (De Jong et al., 2004; Cieplik et al., 2006). However, such conversions require the presence of active metals, as pure silica or aluminas do not yield a significant conversion of precursors (Mosallanejad et al., 2016, 2015, 2014).
It has been clearly demonstrated that CPh are intermediates in essentially all pathways of PCDD/F formation (Dickson et al., 1992; Altwicker and Milligan, 1993; Karasek and Dickson, 1987). Similarly, CBz have been experimentally established as potent precursors forming PCDD/F (Addink and Olie, 1995; Stanmore, 2004) and are also among the most abundant aromatic compounds in incinerator flue gas (Zimmermann et al., 2001). In addition, much of previous research on PCDD/F formation via the precursor pathway was using either single CPh or mixtures of CPh (Sidhu et al., 1995; Born et al., 1993; Ryu et al., 2005). However, virtually no studies have been reported to demonstrate the potential contribution and subsequent influence on PCDD/F emissions from a mixture of CPh and CBz in varying molar concentrations. According to the precursor model, surface catalysed PCDD/F formation proceeds according to two different schemes: the Langmuir-Hinshelwood (L-H) mechanism that involves the reaction of two adsorbed surface species and the Eley-Rideal (E-R) mechanism in which a gas phase species reacts with another surface-bound adsorbed species. For reactions of molecular precursors we have previously demonstrated that PCDF are formed in accord to the L-H mechanism, whereas the formation of PCDD proceeds through an E-R mechanism (Lomnicki and Dellinger, 2003a, 2003b). Both are initiated by chemisorption of substituted aromatic species to metal oxide or hydroxide surface sites, forming phenoxyl-type surface radicals that subsequently undergo self-condensation to form dioxins and other reaction products (Lomnicki et al., 2008; Dellinger et al., 2007).
Our present studies strengthen the thesis that the ratio of CBz to CPh determines the PCDD/PCDF ratio. In here, we report the results of a PCDD/F formation over iron (III) oxides starting from of a mixture of 2-MCP and 1,2-DCBz, with a varying relative gas phase composition and a constant total concentration, in both pyrolytic and oxygen rich conditions. These studies provide an important insight on the possible origin of PCDD/F formation and emission and question the validity of de novo contribution to PCDD/F emissions.
2 Experimental
The surface-mediated reactions of a mixture of 2-MCP and 1,2-DCBz in a molar ratio of 1:10, 1:1 and 10:1 and a total concentration of 50 ppm were studied over Fe2O3/silica surfaces using the System of Thermal Diagnostic Studies (STDS), described elsewhere (Rubey and Grant, 1988). Briefly, this system is composed of a thermal reactor placed in a high-temperature furnace featuring precise temperature control as well as easy sample introduction. A computer-interface control module is used to set and monitor all experimental parameters. A high resolution GC-MS inline with the outlet of the thermal reactor ensures the chemical analysis of the reaction effluent.
The method of incipient wetness was used to prepare the catalytic material serving as surrogate for spontaneously combustion-generated iron-rich fly-ash. Silica gel powder (Aldrich, grade 923 100–200 mesh size) was introduced into an aqueous solution of iron (III) nitrate (Aldrich) in an amount for incipient wetness to occur and a proportion to produce 5% by weight of Fe2O3 in silica. The sample aged for 24 h at room temperature and dried then at 120°C for 12 h before its calcination in air for 5 h. at 450°C. The samples were ground and sieved to a size of 100–120 mesh, or a particle size of 120–150 µm and a surface area of 450 m2/g.
50 mg of catalytic material was placed between two quartz wool plugs situated in a 3 mm i.d. fused silica reactor placed in the STDS. All transfer lines were maintained at a constant temperature of 180°C to avoid the condensation of the reaction products. Prior to each experiment, the catalytic material was oxidised in situ at 450°C for 1 h at an air flow-rate of 5 cc/min, to activate the surface of the sample. The three mixture samples of 2-MCP (Aldrich) and 1,2-DCBz (Aldrich) were introduced separately into the flow stream using a digital syringe pump (KD Scientific, Model-100) through a vaporiser maintained at 180 °C. Nitrogen was used as a carrier gas, and the rate of injection was selected so as to maintain a constant concentration of 50 ppm of (2-MCP + 1,2-DCBz) at temperatures ranging from 200 to 550°C. The overall flow rate of the reaction gas stream was maintained at 5 cc/min. The residence time in the catalytic bed was 0.02 s, the reaction time 1 h, with constant feed throughout. Oxygen inlet concentration was either 0 or 20%, for pyrolysis and oxidation conditions, respectively.
The reaction products were analysed using an inline Agilent 6890 GC-MSD system. a 30 m, 0.25 mm i.d., 0.25 µm film thickness column was used (Restek RTS 5MX) with a temperature hold at −60°C for the reaction period, followed by a temperature program from −60 to 300°C at 10°C /min. Detection and quantification of the products were obtained using an Agilent 5973 mass spectrometer operated in the full-scan mode from 15–350 amu for the duration of the GC run. Products were identified based on mass spectra of the standards, comparisons to the NIST mass spectra library when available, and gas-chromatographic retention times.
The product yields were calculated by the following expression:
where [product] is the specific product concentration formed (in moles) and [2-MCP + 1,2-DCBz]o the initial concentration of 2-MCP + 1,2-DCBz mixtures (in moles) injected into the reactor. Each experimental data point is an average result of three experimental runs. Quantitative standards were used to calibrate the MS response for all products. The reactor was periodically cleaned by heating in air at 800°C and blank runs were routinely performed to assure the absence of deposits or carryover of reaction products from run to run. Presented data does not include analysis of catalytic material for the content of adsorbed products as we focused entirely on the gas phase products.
3 Results and discussion
The catalytic reaction on the surface of fly ash typically occurs within the cooling zone of the incinerator, at temperatures between 150–500°C, in which the yield of PCDD/F is the highest. Upstream of this cooling zone, poor mixing and large catalytic surfaces can result in local oxygen deficiency causing pyrolytic pockets, where reactions occur in oxygen-starved conditions. This is particularly important for the formation of PCDD/F, as oxygen concentration affects both the PCDD/F yield and the PCDD/PCDF ratio (Altarawneh et al., 2009). The catalytic conversion of PCDD/F precursors such as CBz and CPh can also be affected by oxygen concentration, as precursor oxidation is the main pathway of reaction and as lattice oxygen of CuO or Fe2O3 catalytic surfaces drives the oxidation of precursors, proceeding according to a Mars-van Krevelen mechanism (Nganai et al., 2012).
Similar trends were observed in the case of 2-MCP and 1,2-DCBz mixtures, the subject of current studies. The decomposition profiles of 1,2-DCBz show a very similar behaviour for both pyrolytic and oxygen-rich conditions [cf., Figure 1(a) and Figure 1(c), respectively], without any dramatic changes between both reaction conditions. However, the introduction of oxygen surges the degradation yield of 1,2-DCBz, with the biggest increase observed for the experiments with a 2-MCP/1,2-DCBz ratio of 0.1, corresponding with a 10-fold excess of 1,2-DCBz. In the case of 2-MCP, the presence of oxygen improves the degradation yield [cf., Figure 1(b) and Figure 1(d)] of this PCDD/F precursor, and the difference is moderate.
Figure 1.
Degradation profile of the precursors at oxygen rich (A,B) and pyrolytic (C,D) conditions over iron(III) oxide systems
These data again confirm earlier findings, that lattice oxygen is the active species involved in the reaction. The rise of the degradation yield upon the introduction of oxygen can be attributed to surface replenishment with oxygen. The yield increase with reaction temperature, on the other hand, results from the activation of active centres (or surface oxygen species) with higher activation barrier.
Reaction of two different compounds on the catalytic surface is often subject to competitive adsorption. In fact, the present data indicate strong competition between CPh and CBz. For both pyrolytic and oxygen-rich conditions and a 2-MCP/1,2-DCBz ratio of 0.1, the 2-MCP conversion exceeds that of 1,2-dichlorobenzenes over almost the entire temperature range. Pure 1,2-DCBz pyrolysis over the same catalytic surface shows a larger destruction of 1,2-DCBz over the entire temperature range, compared to precursor experiments with 10% addition of 2-MCP (2-MCP / 1,2-DCBz = 0.1) [Figure 1(a)]; previously reported conversion data of 2-MCP over iron oxide/silica surrogate samples have shown similar 2-MCP conversion to the ones observed for mixtures (Nganai et al., 2012). In oxygen-rich conditions, the degradation profile of 1,2-DCBz is not affected by a small addition of 2-MCP to the stream (2-MCP/1,2-DCBz of 0–0.1 – cf. Figure 1(c). These observations strongly support the earlier hypothesis that competitive adsorption between CBz and CPh may play a crucial role in the mechanism of PCDD/F formation in mixed streams. In the case of pyrolytic conditions, the number of available surface sites is limited, owing to the consumption of surface oxygen during the reaction and the competition for the sites between 2-MCP and 1,2-DCBz results in a decreasing 1,2-DCBz conversion. Once sufficient amounts of gas phase oxygen are present to regenerate reacted sites, this competition effect becomes less important.
As seen in Figure 1, the decomposition profiles of 2-MCP shift towards lower temperatures, when compared to 1,2-DCBz, since 2-MCP has two potential pathways of breakdown (water vs. HCl elimination) whereas 1,2-DCBz always chemisorbs through HCl elimination (scheme 1); this results from a difference between the energetics of H2O elimination vs. HCl elimination. the observed results lead to the conclusion that the energetics of the surface reaction of these competing processes requires larger net energy inputs for the reaction through C-Cl bond dissociation compared to the rupturing of the C-OH bond, when assisted by the active surface of iron oxide.
The different reactivity of the two precursors over the surface of iron oxide is also reflected in the yield of PCDD and PCDF. Figures 2 and 3 present the PCDD/F formation profiles as a function of both temperature and the relative concentration of 2-MCP in 2-MCP/1,2-DCBz mixtures (100% corresponds to pure 2-MCP feed and 0% to pure 1,2-DCBz feed) in pyrolytic and oxygen-rich conditions, respectively.
Figure 2.
PCDD/F yields from the pyrolysis reaction of 2-MCP and 1,2-DCBZ mixtures over iron oxide (see online version for colours)
Figure 3.
PCDD/Fs yield form the oxidation of 2-MCP and 1,2-DCBz mixtures over an iron oxide catalytic system (see online version for colours)
The simple condensation products of both 1,2-DCBz and 2-MCP are non-chlorinated dibenzo-p-dioxin (DD), 1-monochloropdibenzo-p-dioxin (MCDD) and 4,6-dichlorodibenzofuran (DCDF) (Nganai et al., 2011, 2012). Within these studies, non-chlorinated PCDD/F products are considered as a representative group of PCDD/F formed by a condensation process, accompanied by the elimination of 2-chlorine atoms. Since 2-MCP and 1,2-DCBz are used in these test reactions, the 2-chlorine elimination process results in the formation of non-chlorinated products, while higher chlorinated precursors will yield the corresponding chlorinated dioxins and furans, still with decreased number of chlorine atoms. In both pyrolytic and oxygen-rich conditions the formation of PCDD congeners is a function of the content of 2-MCP in the feed stream. For pyrolysis the highest yields of PCDD congeners were formed in a stream composed of 90% 2-MCP and 10% 1,2-DCBz. The only exception observed was for the 50% 2-MCP stream, where DD formation was the highest.
This trend was even more prominent for oxygen-rich conditions, where the maximum PCDD formation was significantly higher compared to the PCDF yields (Figure 3). However the formation window of PCDD is very narrow. Also in this case, with increasing 1,2-DCBz content, PCDF yields were increasing, with the highest yields between 90%–50% 2-MCP in stream.
It is difficult to compare the yields of specific congeners, when their formation window is very narrow and does not coincide. In such cases the maximum formation yield might be deceiving, as the effective congener output from combustion sources would correspond to the formation within the whole cooling zone. It is thus much more relevant to compare the average formation yields within the whole temperature range, where the average is defined as integrated yield divided by the number of integrations (or temperature points):
Figure 4 presents distribution of all four observed congeners for each precursor mixture for both pyrolytic and oxygen rich conditions. For both pyrolytic and oxygen rich conditions the trends of congener distribution are generally the same. In general, with increasing content of 1,2-DCBz, the chlorination degree of PCDD/Fs is increasing. This increase is generally a result of increased formation of DCDF. Interestingly, we did not see formation of higher chlorinated PCDD/Fs on iron oxide, indicating that primarily the PCDD/Fs were formed due to the precursor condensation without the surface assisted chlorination.
Figure 4.
Congener distribution for the integrated PCDD/Fs yields over iron oxide sample (see online version for colours)
Figure 5 presents the integrated average yields of PCDD/F for the mixtures of 2-MCP and 1,2-DCBz and, for comparison, data published before for PCDD/F formation over CuO surfaces, determined at the same experimental conditions (Nganai et al., 2014). Based on Figure 5, it is apparent that with decreasing 2-MCP content in the feed stream, the PCDF formation yield is increasing and the plateau at the level of ~0.4% for pyrolytic and ~0.6% for oxidative conditions. A pure 1,2-DCBz feed results in overall lower yields, as 1,2-DCBz is oxidised efficiently due to the lack of competitive adsorbent. PCDD, on the other hand, are formed with the highest yield at a 90% 2-MCP content in the feed stream, and decrease to non-detects for a pure 1,2-DCBz stream. It needs to be emphasised, however, that the presented yields are quite arbitrary for the specific reaction conditions (total of 50 ppm of chlorinated aromatics), and with changing total concentrations of reactants the yields may be different. However, we have confirmed a strong trend correlating the relative change in the yield of PCDD and PCDF with the changing composition of feedstock – excess of CPh increase the yield of PCDD, while CBz control the yields of PCDF. Indeed we have observed this behaviour for the two most prominent metal oxides responsible for the formation of PCDD/F in the cool zone of incinerators, copper and iron oxides.
Figure 5.
Integrated yields of total PCDDs and total PCDFs for (a) pyrolysis (b) oxidation conditions and varying 2-MCP/1.2-DCBz composition of feed gases, in the presence of either iron (green/brown bars) or copper oxides (blue/red bars) (see online version for colours)
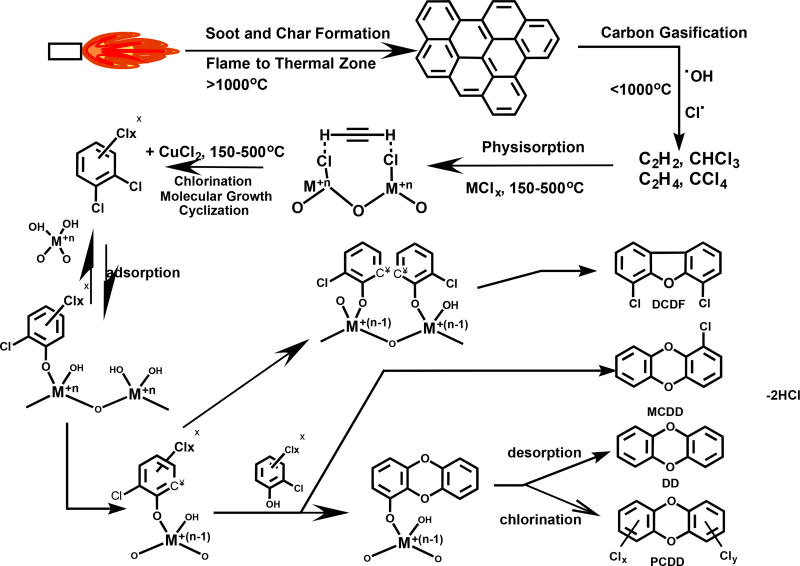
4 Study implications – precursor vs. de novo
As stated in the introduction it is commonly assumed that low PCDD/PCDF ratios indicate the prominence of de novo PCDD/F formation. A significant shortcoming of the de novo model is the assumption of the incorporation within the carbonaceous matrix of dioxin-like structures, ready to be expelled to the gas phase upon the thermal dissociation of the many C-C bonds that hold the structure within the matrix. Most de novo researchers attribute this process to active metal chlorides acting as catalysts in the process (cf., Figure 6). Indeed studies have shown that oxygen concentration has no effect on the PCDD/F formation during de novo synthesis, confirming the thermal nature of the C-C bond scission (Wikström et al., 2003a).1 On the other hand, studies have also shown that the presence of chlorine only results in the formation of C1-C4 chlorinated species (Taylor and Dellinger, 1999). The presence of metal oxide/chlorides is thus necessary for the PCDD/F formation within the de novo model (Wikström et al., 2003b).
Figure 6.
General view of the de novo formation of PCDD/Fs
Interestingly, studies of de novo formation of PCDD/F indicated the formation of CBz and CPh, with yields of CBz a ~100 fold higher compared to CPh (Hell et al., 2001) and ~100 fold higher than the amount of formed PCDD/F. The same proportions of CBz, CPh and PCDD/F were detected during experiments using 1,2-DCBz as a precursor over copper and iron oxide systems (Nganai et al., 2014, 2012). This leads to the following assumption: is it possible that de novo formation as described in Figure 6 pays no contribution to the overall PCDD/F emission? Instead, do the thermal and catalytic oxidation reactions of carbonaceous materials produce dioxin precursor molecules, which undergo the surface condensation reaction according to the precursor model? (cf., Figure 7).
Figure 7.
Unified PCDD/F formation model as proposed by Dellinger and Lomnicki (see online version for colours)
The present results confirm that the PCDD/PCDF-ratio cannot be used to validate the de novo pathway nor can it be used as an indicator of the de novo reaction in incinerators. In fact, the PCDD/PCDF-ratio is strongly dependent on the relative concentrations of precursor groups in the reaction stream – CBz vs. CPh. Figure 8 illustrates this dependence for iron oxide systems, and a similar correlation was presented before for copper oxide.
Figure 8.
PCDD/PCDF-ratio as a function of the 2-MCP content in the 2-MCP/1,2-DiCBz reaction stream (see online version for colours)
The model presented here is based on experimental data of a simple, specific system designed to mimic reactions on real fly ash. In fact, real fly ash samples at the boiler section of combustors and incinerators contain a very complex mix of matrix materials and many metals speciated as oxides, chlorides, sulphates, sulphides, etc. Some of these are formed during cooling and de-sublimation, or else after reporting to boiler deposits. They also contain occluded polyaromatic materials, as well as some adsorbed precursors. So, the presented data cannot span the entire range of possible fly ash systems, yet this contribution hopes to contribute to opening the discussion on relative roles for precursors and de novo.
To conclude, the contribution of de novo PCDD/F formation should be revisited and laboratory experiments need to be designed very precisely to establish the role of the pure de novo reaction and distinguish it from the secondary precursor pathway resulting from precursors emitted during the oxidation of carbonaceous fractions. This should also include the role of other organic components in the gas stream as they may result in the formation of CPh and benzenes precursors through surface assisted reactions.
Acknowledgments
This research was supported by National Institute of Environmental Health Sciences, grant # 2P42 ES013648.
Biographies
Slawo (Slawomir) Lomnicki has a PhD in Physical Chemistry from A. Mickiewicz University in Poznan, Poland. He had his Post-Doctoral training in Environmental Chemistry and Catalysis at the University of South Carolina, Department of Chemical Engineering and Louisiana State University, Department of Chemistry. He became a Research Professor at LSU, where he further worked on emission from combustion systems, including dioxins, particulate matter and environmentally persistent free radicals. He is also a Project Leader and Materials Core Director at the LSU Superfund Research Center. At present, he is an Assistant Professor at the Department of Environmental Sciences, Louisiana State University.
Shadrack Nganai received his PhD from Louisana State University under the supervision of Dr. Barry Dellinger and Dr. Slawo Lomnicki. At present, he is a Lecturer at South Louisiana Community College.
Footnotes
These tests are in disagreement with careful work from both Stieglitz and Addink. Wikström used an experimental design to study 20 variables in 10 experiments!
Contributor Information
Shadrack Nganai, Chemistry Department, South Louisiana Community College, Lafayette, LA, USA.
Slawo Lomnicki, Department of Environmental Sciences, Louisiana State University, Baton Rouge, LA, USA.
References
- Addink R, Olie K. Mechanisms of formation and destruction of polychlorinated dibenzo-p-dioxins and dibenzofurans in heterogeneous systems. Environmental Science and Technology. 1995;29(6):1425–1435. doi: 10.1021/es00006a002. [DOI] [PubMed] [Google Scholar]
- Altarawneh M, Dlugogorski BZ, Kennedy EM, Mackie JC. Mechanisms for formation, chlorination, dechlorination and destruction of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) Progress in Energy and Combustion Science. 2009;35(3):245–274. [Google Scholar]
- Altwicker ER, Milligan MS. Formation of dioxins – competing rates between chemically similar precursors and de-novo reactions. Chemosphere. 1993;27(1–3):301–307. [Google Scholar]
- Altwicker ER, Schonberg JS, Konduri R, Milligan MS. Polychlorinated dioxin furan formation in incinerators. Hazardous Waste and Hazardous Materials. 1990;7(1):73–87. [Google Scholar]
- Born JGP, Mulder P, Louw R. Fly-ash mediated reactions of phenol and monochlorophenols – oxychlorination, deep oxidation, and condensation. Environmental Science and Technology. 1993;27(9):1849–1863. [Google Scholar]
- Brzuzy LP, Hites RA. Global mass balance for polychlorinated dibenzo-p-dioxins and dibenzofurans – response. Environmental Science and Technology. 1996;30(12):3647–3648. doi: 10.1021/es00008a031. [DOI] [PubMed] [Google Scholar]
- Cains PW, McCausland LJ, Fernandes AR, Dyke P. Polychlorinated dibenzo-p-dioxins and dibenzofurans formation in incineration: effects of fly ash and carbon source. Environmental Science and Technology. 1997;31(3):776–785. [Google Scholar]
- Cieplik MK, De Jong V, Bozovic J, Liljelind P, Marklund S, Louw R. Formation of dioxins from combustion micropollutants over MSWI fly ash. Environmental Science and Technology. 2006;40(4):1263–1269. doi: 10.1021/es052225l. [DOI] [PubMed] [Google Scholar]
- De Jong V, Cieplik NK, Louw R. Formation of dioxins in the catalytic combustion of chlorobenzene and a micropollutant-like mixture on Pt/gamma-Al2O3. Environmental Science and Technology. 2004;38(19):5217–5223. doi: 10.1021/es034820y. [DOI] [PubMed] [Google Scholar]
- Dellinger B, Loninicki S, Khachatryan L, Maskos Z, Hall RW, Adounkpe J, McFerrin C, Truong H. Formation and stabilization of persistent free radicals. Proceedings of the Combustion Institute. 2007;31:521–528. doi: 10.1016/j.proci.2006.07.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson LC, Lenoir D, Hutzinger O. Surface-catalyzed formation of chlorinated dibenzodioxins and dibenzofurans during incineration. Chemosphere. 1989;19(1–6):277–282. [Google Scholar]
- Dickson LC, Lenoir D, Hutzinger O. Quantitative comparison of Denovo and precursor formation of polychlorinated dibenzo-p-dioxins under simulated municipal solid-waste incinerator postcombustion conditions. Environmental Science and Technology. 1992;26(9):1822–1828. [Google Scholar]
- Environmental Protection Agency. Database of Sources of Environmental Releases of Dioxin – Like Compounds in the United State, Report EPA/600/C-01/012. USA: 2001. [Google Scholar]
- Evans CS, Dellinger B. Surface-mediated formation of polybrominated dibenzo-p-dioxins and dibenzofurans from the high-temperature pyrolysis of 2-bromophenol on a CuO/silica surface. Environmental Science and Technology. 2005;39(13):4857–4863. doi: 10.1021/es048057z. [DOI] [PubMed] [Google Scholar]
- Fiedler H. Thermal formation of PCDD/PCDF: a survey. 1998:49–58. [Google Scholar]
- Ghorishi SB, Altwicker ER. Formation of polychlorinated dioxins, furans, benzenes and phenols in the postcombustion region of a heterogeneous combustor – effect of bed material and postcombustion temperature. Environmental Science and Technology. 1995;29(5):1156–1162. doi: 10.1021/es00005a004. [DOI] [PubMed] [Google Scholar]
- Hell K, Stieglitz L, Dinjus E. Mechanistic aspects of the de-novo synthesis of PCDD/PCDF on model mixtures and MSWI fly ashes using amorphous C-12- and C-13-labeled carbon. Environmental Science and Technology. 2001;35(19):3892–3898. doi: 10.1021/es0100266. [DOI] [PubMed] [Google Scholar]
- Huang H, Buekens A. On the mechanisms of dioxin formation in combustion processes. Chemosphere. 1995;31(9):4099–5117. [Google Scholar]
- Ismo H, Kari T, Juhani R. Formation of aromatic chlorinated compounds catalyzed by copper and iron. Chemosphere. 1997;34(12):2649–2662. [Google Scholar]
- Karademir A, Korucu MK. Assessment of emissions and removal of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) at start-up periods in a hazardous waste incinerator. Journal of the Air and Waste Management Association. 2013;63(7):788–795. doi: 10.1080/10962247.2013.790856. [DOI] [PubMed] [Google Scholar]
- Karasek FW, Dickson LC. Model studies of polychlorinated dibenzo-para-dioxin formation during municipal refuse incineration. Science. 1987;237(4816):754–756. doi: 10.1126/science.3616606. [DOI] [PubMed] [Google Scholar]
- Lomnicki S, Dellinger B. A detailed mechanism of the surface-mediated formation of PCDD/F from the oxidation of 2-chlorophenol on a CuO/silica surface. Journal of Physical Chemistry A. 2003a;107(22):4387–4395. [Google Scholar]
- Lomnicki S, Dellinger B. Formation of PCDD/F from the pyrolysis of 2-chlorophenol on the surface of dispersed copper oxide particles. Proceedings of the Combustion Institute. 2003b;29:2463–2468. [Google Scholar]
- Lomnicki S, Vajerano E, Wu HY, McCarley RL, Poliakoff ED, Dellinger B. ANYL 342-Chemical Activity of the Combustion Generated Nanoparticles; Abstracts of papers of the American Chemical Society; 2008. p. 236. [Google Scholar]
- Milligan MS, Altwicker ER. Chlorophenol reactions on fly ash .1: adsorption desorption equilibria and conversion to polychlorinated dibenzo-p-dioxins. Environmental Science and Technology. 1996;30(1):225–229. [Google Scholar]
- Mosallanejad S, Dlugogorski BZ, Altarawneh M, Kennedy EM, Stockenhuber M. Comparison of decomposition of 2-chlorophenol on surfaces of alumina- and silica-supported iron (III) oxide catalysts. Organohalogen Compd. 2015;77:210–213. [Google Scholar]
- Mosallanejad S, Dlugogorski BZ, Altarawneh M, Kennedy EM, Yokota M, Nakano T, Stockenhuber M. Decomposition of 2-chlorophenol on surfaces of neat alumina and alumina supported iron (III) oxide catalysts. Organohalogen Compd. 2014;76:396–399. [Google Scholar]
- Mosallanejad S, Dlugogorski BZ, Kennedy EM, Stockenhuber M, Lomnicki SM, Assaf NW, Altarawneh M. Formation of PCDD/Fs in oxidation of 2-chlorophenol on neat silica surface. [accessed 2016];Environmental Science and Technology. 2016 50(3):1412–1418. doi: 10.1021/acs.est.5b04287. http://pubs.acs.org/doi/pdfplus/10.1021/acs.est.5b04287 [online] http://dx.doi.org/10.1021/acs.est.5b04287 ; ; http://pubs.acs.org/doi/pdfplus/10.1021/acs.est.5b04287. [DOI] [PubMed] [Google Scholar]
- Nganai S, Dellinger B, Lomnicki S. PCDD/PCDF ratio in the precursor formation model over CuO surface. Environmental Science and Technology. 2014;48(23):13864–13870. doi: 10.1021/es504253w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nganai S, Lomnicki S, Dellinger B. Formation of PCDD/Fs from oxidation of 2-monochlorophenol over an Fe2O3/silica surface. Chemosphere. 2012;88(3):371–376. doi: 10.1016/j.chemosphere.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nganai S, Lomnicki SM, Dellinger B. Formation of PCDD/Fs from the copper oxide-mediated pyrolysis and oxidation of 1,2-dichlorobenzene. Environmental Science and Technology. 2011;45(3):1034–1040. doi: 10.1021/es102948f. [DOI] [PubMed] [Google Scholar]
- Olie K, Addink R, Schoonenboom M. Metals as catalysts during the formation and decomposition of chlorinated dioxins and furans in incineration processes. Journal of the Air and Waste Management Association. 1998;48(2):101–105. doi: 10.1080/10473289.1998.10463656. [DOI] [PubMed] [Google Scholar]
- Rubey WA, Grant RA. Design aspects of a modular instrumentation system for thermal diagnostic studies. Review of Scientific Instruments. 1988;59(2):265–269. [Google Scholar]
- Ryu JY, Mulholland JA, Kim DH, Takeuchi M. Homoloque and isomer patterns of polychlorinated dibenzo-p-dioxins and dihenzofurans from phenol precursors: comparison with municipal waste incinerator data. Environmental Science and Technology. 2005;39(12):4398–4406. doi: 10.1021/es048224v. [DOI] [PubMed] [Google Scholar]
- Sidhu SS, Maqsud L, Dellinger B, Mascolo G. The homogeneous, gas-phase formation of chlorinated and brominated dibenzo-p-dioxin from 2,4,6-trichlorophenols and 2,4,6-tribromophenols. Combustion and Flame. 1995;100(1–2):11–20. [Google Scholar]
- Stanmore BR. The formation of dioxins in combustion systems. Combustion and Flame. 2004;136:368–427. [Google Scholar]
- Stieglitz L. Selected topics on the de novo synthesis of PCDD/PCDF on fly ash. Environmental Engineering Science. 1998;15(1):5–18. [Google Scholar]
- Stieglitz L, Zwick G, Beck J, Roth W, Vogg H. On the de-novo synthesis of PCDD/PCDF on fly-ash of municipal waste incinerators. Chemosphere. 1989;18(1–6):1219–1226. [Google Scholar]
- Stieglitz LVH, Zwick G, Beck J, Bautz H. On formation conditions of organohalogen compounds from particulate carbon of fly ash. Chemosphere. 1991;23(8–10):1255–1264. [Google Scholar]
- Taylor PH, Dellinger B. Pyrolysis and molecular growth of chlorinated hydrocarbons. Journal of Analytical and Applied Pyrolysis. 1999;49(1–2):9–29. [Google Scholar]
- Thomas VM, Spiro TG. The US dioxin inventory: are there missing sources? Environmental Science and Technology. 1996;30(2):A82–A85. doi: 10.1021/es962098g. [DOI] [PubMed] [Google Scholar]
- Vermeulen I, Van Caneghem J, Vandecasteele C. Indication of PCDD/F formation through precursor condensation in a full-scale hazardous waste incinerator. Journal of Material Cycles and Waste Management. 2014;16(1):167–171. [Google Scholar]
- Wikström E, Ryan S, Touati A, Gullett BK. Key parameters for de novo formation of polychlorinated dibenzo-p-dioxins and dibenzofurans. Environmental Science and Technology. 2003a;37(9):1962–1970. doi: 10.1021/es026240r. [DOI] [PubMed] [Google Scholar]
- Wikström E, Ryan S, Touati A, Telfer M, Tabor D, Gullett BK. Importance of chlorine speciation on de Novo formation of polychlorinated dihenzo-p-dioxins and polychlorinated dibenzofurans. Environmental Science and Technology. 2003b;37(6):1108–1113. doi: 10.1021/es026262d. [DOI] [PubMed] [Google Scholar]
- Xiao X, Hu JF, Chen P, Chen DY, Huang WL, Peng PA, Ren M. Spatial and temporal variation, source profile, and formation mechanisms of PCDD/Fs in the atmosphere of an e-waste recycling area, South China. Environmental Toxicology and Chemistry. 2014;33(3):500–507. doi: 10.1002/etc.2460. [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Blumenstock M, Heger HJ, Schramm KW, Kettrup A. Emission of nonchlorinated and chlorinated aromatics in the flue gas of incineration plants during and after transient disturbances of combustion conditions: delayed emission effects. Environmental Science and Technology. 2001;35(6):1019–1030. doi: 10.1021/es000143l. [DOI] [PubMed] [Google Scholar]