Abstract
Multifocal atrial tachycardia (MAT) has a well-known association with Costello syndrome, but is rarely described with related RAS/MAPK pathway disorders (RASopathies). We report 11 patients with RASopathies (Costello, Noonan, and Noonan syndrome with multiple lentigines [formerly LEOPARD syndrome]) and nonreentrant atrial tachycardias (MAT and ectopic atrial tachycardia) demonstrating overlap in cardiac arrhythmia phenotype. Similar overlap is seen in RASopathies with respect to skeletal, musculoskeletal and cutaneous abnormalities, dysmorphic facial features, and neurodevelopmental deficits. Nonreentrant atrial tachycardias may cause cardiac compromise if sinus rhythm is not restored expeditiously. Typical first-line supraventricular tachycardia anti-arrhythmics (propranolol and digoxin) were generally not effective in restoring or maintaining sinus rhythm in this cohort, while flecainide or amiodarone alone or in concert with propranolol were effective anti-arrhythmic agents for acute and chronic use. Atrial tachycardia resolved in all patients. However, a 4-month-old boy from the cohort was found asystolic (with concurrent cellulitis) and a second patient underwent cardiac transplant for heart failure complicated by recalcitrant atrial arrhythmia. While propranolol alone frequently failed to convert or maintain sinus rhythm, fleccainide or amiodarone, occasionally in combination with propranolol, was effective for RASopathy patient treatment for nonreentrant atrial arrhythmia. Our analysis shows that RASopathy patients may have nonreentrant atrial tachycardia with and without associated cardiac hypertrophy. While nonreentrant arrhythmia has been traditionally associated with Costello syndrome, this work provides an expanded view of RASopathy cardiac arrhythmia phenotype as we demonstrate mutant proteins throughout this signaling pathway can also give rise to ectopic and/or MAT.
Keywords: Calcium, Costello syndrome, ectopic atrial tachycardia, multifocal atrial tachycardia, Noonan syndrome, Noonan syndrome with multiple lentigines, RAS/MAPK signaling pathway
1| INTRODUCTION
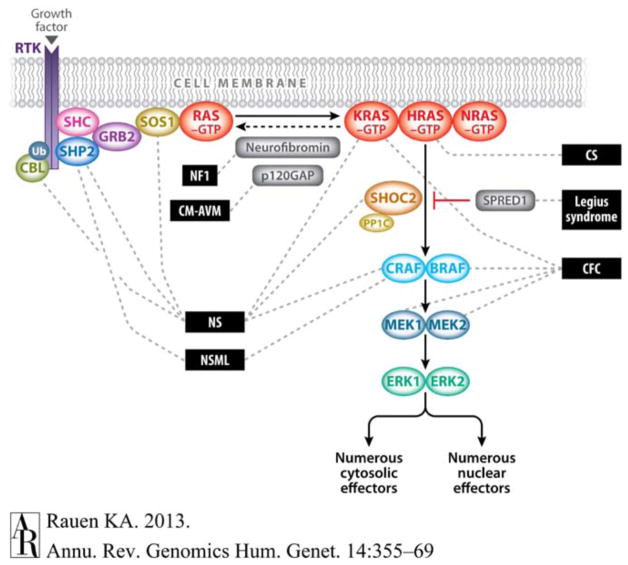
The RAS/MAPK signaling pathway performs critical cellular differentiation and maintenance functions in a variety of organ systems (Figure 1) (Rauen, 2013; Tidyman & Rauen, 2009). Activating mutations in the RAS/MAPK signaling pathway result in a family of related disorders termed “RASopathies” that include Costello syndrome (OMIM #218040), Noonan syndrome (#163950), Noonan syndrome with multiple lentigines (formerly known as LEOPARD syndrome) (#151100), and Cardiofaciocutaneous syndrome (#115050). This group of disorders can have overlapping skeletal, dermatologic, musculoskeletal, and neurocognitive features. Additionally, individuals with RASopathies frequently express similar cardiac phenotypes, encompassing structural and electrophysiologic abnormalities. Common cardiac abnormalities seen in RASopathies include hypertrophic cardiomyopathy (HCM), valve abnormalities, particularly pulmonic valve stenosis, and dysplasia, as well as an increased incidence of atrial and ventricular septal defects among others (Lin et al., 2011; Prendiville et al., 2014). While the frequency and type of structural cardiovascular abnormalities in RASopathies have been detailed elsewhere (Lin et al., 2011; Pandit et al., 2007; Pierpont et al., 2014; Prendiville et al., 2014; Rauen et al., 2010; van Berlo, Maillet, & Molkentin, 2013), cardiac electrophysiological abnormalities in these syndromes have been less well characterized (Dodo, Gow, Hamilton, & Freedom, 1995), with the exception of individuals with Costello syndrome who have been described with multifocal atrial tachycardia (MAT). Although much progress has been made in identifying the genetic causes of heritable arrhythmias, the genetic basis of nonreentrant atrial arrhythmias in infants and children is largely unknown. An attempt to identify genes that are known to cause triggered activity, and are not already known to cause channelopathy (long QT syndrome [LQTS], catecholaminergic polymorphic ventricular tachycardia [CPVT], or Brugada Syndrome) or familial atrial fibrillation yields a surprisingly small number of genes. Such genes include guanine nucleotide-binding protein inhibiting polypeptide (GNAI2, OMIM #192605); and trans-2,3-enoyl-CoA reductase-like protein (TERCL, OMIM #617242), which appears to regulate two proteins known to cause CPVT, the ryanodine receptor (RYR2, OMIM# 180902) and calsequestrin (CASQ2, OMIM#114251) . One gene associated with both ectopic and MAT includes troponin I interacting kinase (TNNI3K, OMIM#613932), a kinase that is also thought to affect calcium handling, and is associated with a cardiomyopathy phenotype in some instances (Theis et al., 2014). The only other two genes associated with these arrhythmias identified in OMIM are those in the RAS/MAPK pathway (HRAS, KRAS, NRAS, OMIM #190020, OMIM #218040).
FIGURE 1.
The RAS/mitogen-activated protein kinase (MAPK) signaling pathway and associated developmental syndromes (indicated by dashed lines). The MAPK signaling pathway of protein kinases is critically involved in cell proliferation, differentiation, motility, apoptosis, and senescence. The RAS/MAPK pathway proteins with germline mutations in their respective genes are associated with Noonan (NS), Noonan syndrome with multiple lentigines (NSML)(LEOPARD), neurofibromatosis, type 1 (NF1), capillary malformation–arteriovenous malformation (CM-AVM) due to RAS1 mutations, Costello (CS), Cardiofaciocutaneous (CFC) and Legius syndromes. Figure reprinted from Rauen (2013) with permission from Elsevier. [Color figure can be viewed at wileyonlinelibrary.com]
The purpose of this study is to detail the cardiac electrophysiologic phenotypes and to characterize arrhythmia clinical course including treatment seen in individuals with RASopathies. We demonstrate that nonreentrant atrial tachycardias, MAT and ectopic atrial tachycardia (EAT), can arise from gain-of-function mutations in multiple genes giving rise to RAS/MAPK pathway dysregulation. This represents an expanded description of RASopathy cardiac phenotype as nonreentrant arrhythmia had previously only been reported in Costello syndrome patients. We further show that arrhythmia can occur in the presence or absence of cardiac hypertrophy, suggesting that the atrial arrhythmia seen in these patients is not simply resulting from hypertrophic cardiomyopathy pressure load. These findings provide important clinical information for the diagnosis and management of RASopathy patients, and may provide insights into nonreentrant atrial arrhythmia mechanisms.
2| MATERIALS AND METHODS
We retrospectively collected and reviewed the clinical, cardiac, and molecular genetic features of patients with a RASopathy who presented with arrhythmia and/or cardiomyopathy, and were cared for at six institutions (the Children’s Hospital of Philadelphia, A.I. du Pont Hospital for Children, Maine Medical Center, Cincinnati Children’s Hospital Medical Center, Children’s Hospital Colorado, and Sanford Children’s Hospital) between 2008 and 2013. Two-dimensional echocardiography was performed using standard pediatric echocardiographic methods and commercially available machines. Segmental anatomy was confirmed and quantitative measures for left ventricular (LV) end diastolic dimension (LVeDD), LV end-systolic dimension (LVeSD), interventricular septal dimension (IVS) were obtained from m-mode imaging in the parasternal short axis. These values were normalized using gender, age, and body surface area resulting in a z-score. Echocardiography images and reports were reviewed to evaluate for evidence of congenital heart defects, hypertrophic cardiomyopathy, and quantitative wall dimensions. Z-score were extracted and displayed in table format. Standard 12 or 15 lead surface electrocardiograms with rhythm strips were obtained from each patient and reviewed to confirm arrhythmia diagnosis. Of the nonreentrant tachycardias, we defined MAT (also sometimes described as chaotic atrial rhythm, CAR) as a tachyarrhythmia with the following characteristics: (a) multiple (at least three) distinct P-wave morphologies; (b) irregular P-P intervals; (c) isoelectric baseline between P-waves; and (d) ventricular rate >100 beats/min (Bradley, Fischbach, Law, Serwer, & Dick, 2001). EAT is characterized by (a) a P-wave morphology during tachycardia that is unique from the sinus P-wave, (b) the P wave of the first beat is identical to that of subsequent beats in tachycardia; (c) the cycle length of the first few beats may progressively shorten; (d) atrioventricular block can occur in the presence of continuing atrial arrhythmia; and (e) ventricular rate >100 beats/min (Bradley et al., 2001; Salerno, Anderson, Sharkey, & Iber, 1987) .
All patients had a clinical and molecular diagnosis of a RASopathy syndrome. Molecular genetic testing was performed as clinically indicated at the commercial molecular diagnostic labs routinely used by each patient’s institution.
3 | RESULTS
Table 1 summarizes the clinical, molecular and cardiac details of 11 patients with RASopathies, including two patients with Costello syndrome, Noonan syndrome with multiple lentigines (LEOPARD syndrome) (two patients), and Noonan syndrome (seven patients).
TABLE 1.
Genetic, arrhythmia and echocardiographic findings in 11 patients with RASopathies
| Pt no | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | M | F | M | M | M | F | F | M | F | M | F 5 M 6 |
| Genetic and molecular findings | ||||||||||||
|
| ||||||||||||
| Syndrome | CS | CS | NS | NS | NS | NS | NS | NS-ML | NS-ML | NS | NS |
CS 2 NS 7 NS-ML 2 |
|
| ||||||||||||
| Age at disease presentation | Birth | 4 mos | Birth | Birth | Birth | Birth | Birth | Birth, after cardiac eval | Birth after cardiac eval | 2 wks | Birth | |
|
| ||||||||||||
| Affected Gene | HRAS | HRAS | RAF1 | RAF1 | RAF1 | RAF1 | PTPN11 | PTPN11 | PTPN11 | SOS1 | KRAS |
HRAS 2 RAF1 4 PTPN11 3 SOS1 1 KRAS 1 |
|
| ||||||||||||
| Base Change | c.35G>C | c.34G>A | c.770C>T | c.781C>T | c.770C>T | c.770C>T | c.188A>G | c.138G>A | c.1493G>T | c.2536G> A | c.173C>T | |
|
| ||||||||||||
| AA Change | p.Gly12 Ala |
p.Gly12 Ser |
p.Ser257 Leu |
p.Pro261 Ser |
p.Ser257 Leu |
p.Ser257 Leu |
p.Tyr63 Cys |
p.Ala461 Thr |
p.Arg498 Leu |
p.Glu846 Lys |
p.Thr58 Ile |
|
|
| ||||||||||||
| Arrhythmia findings | ||||||||||||
|
| ||||||||||||
| Age at Arrhythmia presentation | Prenatal: 35 wks | Postnatal: 8 mos | Postnatal: 7 days | Postnatal: 1 day | Postnatal: 4 years 1 mo | Postnatal: 3 mos | Postnatal: 2 mos | Postnatal: 3 wks | Postnatal: 2 mos | Postnatal: 4 mos. | Postnatal: 3 wks | Mean: 222 ± 95 days |
|
| ||||||||||||
| MAT | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | Yes | No | 7 Yes 4 No |
|
| ||||||||||||
| EAT | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 Yes 0 No |
|
| ||||||||||||
| SVT | No | Yes | No | No | Yes | Yes | No | No | No | No | Yes | 4 Yes 7 No |
|
| ||||||||||||
| Max arrhythmia rate (presenting ECG (bpm)) | 250 | 240 | 246 | 209 | 220 | 207 | 227 | 190 | 220’s | Atrial rate 300’s, ventricular rate 170 | 300 | Mean: 227.4 ± 37.6 |
|
| ||||||||||||
| Treatment failures | Amio | None | Dig, Prop | Dig, Prop, Amio | Prop, Verap, Sotalol | Prop, Amio thyroid toxicity | Prop | Prop, Amio | Esmolol | Esmolol | None | 6 Prop 2 Esmolol 2 Dig 4 Amio |
|
| ||||||||||||
| Arrhythmia findings | ||||||||||||
|
| ||||||||||||
| Age at Arrhythmia presentation | Prenatal: 35 wks | Postnatal: 8 mos | Postnatal: 7 days | Postnatal: 1 day | Postnatal: 4 years 1 mo | Postnatal: 3 mos | Postnatal: 2 mos | Postnatal: 3 wks | Postnatal: 2 mos | Postnatal: 4 mos. | Postnatal: 3 wks | Mean: 222 ± 95 days |
|
| ||||||||||||
| Treatment | Flec | Prop | Flec | Prop +Flec | Transplant | Propr+ Amio x 2.5 years, Prop + Verap | Prop+ Flec | Flec | Propr +Flec | Amio | Prop | 3 Flec, 2 Prop. 3 Propr +Flec, 1 Amio, 1 Prop+ 1 Amio 1 Prop+ Verap |
|
| ||||||||||||
| Anti-arrhythmic therapy at last FU | Yes | Yes | No | No | NA, after heart tx | Yes | No | No | Yes | No | NA | 4 Yes 4 No |
|
| ||||||||||||
| Duration of anti-arrhythmic therapy (mos) | 24 | 18 | 24 | 16 | 49 | 42 | 36 | 30 | 34 | 6.5 | 4 | Mean: 25.7 ± 14.1 |
|
| ||||||||||||
| Age at last FU | 27 mos (deceased) | 23 mos | 4 yrs | 3 yrs 5 mos |
8 yrs 7 mos |
3 yrs 4 mos | 5 yrs 11 mos |
4 yrs | 3 yrs | 3 yrs 11 mos |
4 mos (deceased) | Mean: 54 ± 27.9mos |
|
| ||||||||||||
| Presenting echocardiogram findings | ||||||||||||
|
| ||||||||||||
| Cardiac hypertrophy at arrhythmia presentation | No | Conc hypertrophy | Conc hypertrophy, with thick septum | No | Mod to severe ASH | ASH | No | ASH | ASH | No | Mild septal hypertrophy |
7 Yes 4 No |
|
| ||||||||||||
| LVeDD Z-score | 2.51 | −1.34 | 2.77 | −1 | −0.54 | −3.58 | 1.3 | 1.43 | −3.84 | −3.9 | −0.63 | |
|
| ||||||||||||
| LVeSD Z-score | 2.03 | −1.76 | 5.32 | −1.16 | −4.56 | −3.64 | 1.4 | 3.43 | −5.02 | −4.1 | −2.0 | |
|
| ||||||||||||
| SF (%) | 38.9% | 45% | 66% | 42.11% | 64% | 46% | 56% | 55% | 55% | 46% | 55.5% | Mean: 51.8 ± 8.8% |
|
| ||||||||||||
| LV mass Z-score | −3.27 | +4.22 | −1.60 | −1.86 | +5.73 | +0.95 | +.25 | +3.23 | 1.79 | NA | +9.42 | |
|
| ||||||||||||
| Valvar PS | No | No | No | Yes | Mild | No | Mild | Mild | No | Severe | Mild | 6 Yes 5 No |
|
| ||||||||||||
| Other CHDs | No | Abn papillary muscle, SAM | No | Mod ASD | No | ASD, small VSD, mild MS | No | No | No | ASD | No | 4 Yes 7 No |
|
| ||||||||||||
| Noncardiac clinical problems | ||||||||||||
|
| ||||||||||||
| Poor feed | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 8 Yes 3 No |
|
| ||||||||||||
| Failure to gain wt | No | No | No | No | No | No | No | No | No | Yes | Yes | 2 Yes 9 No |
|
| ||||||||||||
| Dev. delay | No | Yes | AuSD | Speech delay | Yes | No | Mild | No | No | No | No | 5 Yes 6 No |
|
| ||||||||||||
| Noncardiac clinical problems | ||||||||||||
|
| ||||||||||||
| Typical facies | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | CS-like | 11 Yes ~1 No |
|
| ||||||||||||
| Short stature | No | No | Yes | Yes | Yes | Yes | No | No | No | No | No | 4 Yes 7 No |
|
| ||||||||||||
| Hyper-pigmentation | No | No | CALM | No | No | No | Nevi | CALM | Yes, Lentigines | No | No | 4 Yes 7 No |
|
| ||||||||||||
| Skeletal | No | No | No | Pectus car | No | No | pectus exc | No | No | Macrocephaly | No | |
|
| ||||||||||||
| Other | Embryonic RMS, pelvis | Conductive HL | SS, s/p trach, s/p G-tube | Mildly curly hair | Mild hydronephrosis | |||||||
|
| ||||||||||||
| Fetal Abnormalities | ||||||||||||
|
| ||||||||||||
| Gest age (wks) | 35 | 36 | 38 | 36 | 35 | 33 | 39.5 | 35 | 40 | 39 | 37 | Mean: 36.7 ± 2.2 mos |
|
| ||||||||||||
| Fetal arrhythmia | Yes | None | None | Fetal bradycardia | None | None | NA | None | Yes | No | None | 3 Yes |
|
| ||||||||||||
| Poly-hydramnios | Yes | Yes | Yes | No | No | Yes | NA | Yes | No | Yes | Yes | 7 Yes |
|
| ||||||||||||
| LGA | No | Yes | No | No | No | No | poss SGA | No | No | No | NA | 1 Yes |
|
| ||||||||||||
| Nuchal lucency or cystic hygroma | Yes | No | Yes | No | No | Yes | NA | Yes | No | No | NA | 4 Yes |
|
| ||||||||||||
| Brain abn | VM | No | Macrocelphaly | Retro-cerebellar CSF collection | No | DW | NA | WM atrophy, VM | No | NA | VM | 6 Yes |
|
| ||||||||||||
| Fetal heart disease | Yes Details NA | No | Small muscular VSD | Valvar PS; ASD | Mild valvar PS, abn MV | DORV, hypo MV, AV | NA | Small VSD | No | Valvar PS; ASD | No | 7 Yes |
AA, Amino acid; Abn, abnormal or abnormality; Amio, Amiodarone; ASD, atrial septal defect; ASH, asymmetric septal hypertrophy; AuSD, autistic spectrum disorder; CALM, cafeé au lait macule; car, carinatum; conc, concentric; CS, Costello Syndrome; decd, deceased; Dig, Digoxin; DW, Dandy-Walker malformation; DORV, double outlet right ventricle; EAT, ectopic atrial tachycardia; exc, excavatum; eval, evaluation Flec, Flecainide; FU, follow-up; gest, gestational; HL, hearing loss; hypo, hypoplastic; LGA, large for gestational age; LV, left ventricle; LVeDD, left ventricle end-diastolic dimension; LVeSD, Left ventricle end-systolic dimension; mod, moderate; MS, mitral stenosis; MAT, multi-focal atrial tachycardia; mos, months; NA, not available or not assessed; NS, Noonan syndrome; NS-ML, Noonan syndrome with multiple lentigines; poss, possible; Prop, Propranolol; PS, pulmonary stenosis; Pt no, patient number; RMS, rhabdomyosarcoma; SAM, systolic anterior motion; SS, subglottic stenosis; tx, transplantation; VM, ventriculomegaly; VSD, ventricular septal defect; WM, white matter; wt, weight; wks, weeks; yrs, years; mos, months.
3.1 | Molecular testing
RASopathy phenotypes are seen in patients with missense mutations in HRAS, BRAF, MEK1/2, KRAS, RAF1, PTPN11 (SHP2), SOS1, SHOC2, and CBL, and since the diagnosis of the patients in this series, RIT1. These mutations confer a dominant inheritance pattern, resulting in gain-of-function mutations or activation of the RAS/MAPK pathway (Figure 1) (Pandit et al., 2007; Rauen, 2013; Rodriguez-Viciana et al., 2006; Tartaglia, Cotter, Zampino, Gelb, & Rauen, 2003; Tidyman & Rauen, 2009; Wu et al., 2011), with the exception of Noonan syndrome with multiple lentigines in which there are dominant negative effects from loss-of-function mutations. Each confirmed mutation is listed in Table 1.
3.2 | Noncardiac clinical features
Table 1 summarizes noncardiac and fetal findings where available. Patients were ascertained based on their clinical features. While each syndrome has characteristic clinical features, overlap of the clinical features among syndromes is well-recognized, making it difficult to predict the molecular defect. This is particularly true among Noonan syndrome patients, where mutations in different genes give rise to similar phenotypes (PTPN11, SOS1, RAF1). While cardiac findings featured
3.3 | Cardiac clinical features
Arrhythmias were documented from the fetal period to as late as 4 years of age (mean 222 ± 95 days) (Table 1). Presenting rhythms included MAT and EAT. Among those patients with MAT, EAT was also noted later in their clinical course. Four of these patients demonstrated supraventricular (reentrant) tachycardia in addition to their nonreentrant atrial tachycardia. Ventricular ectopy was present in some individuals.
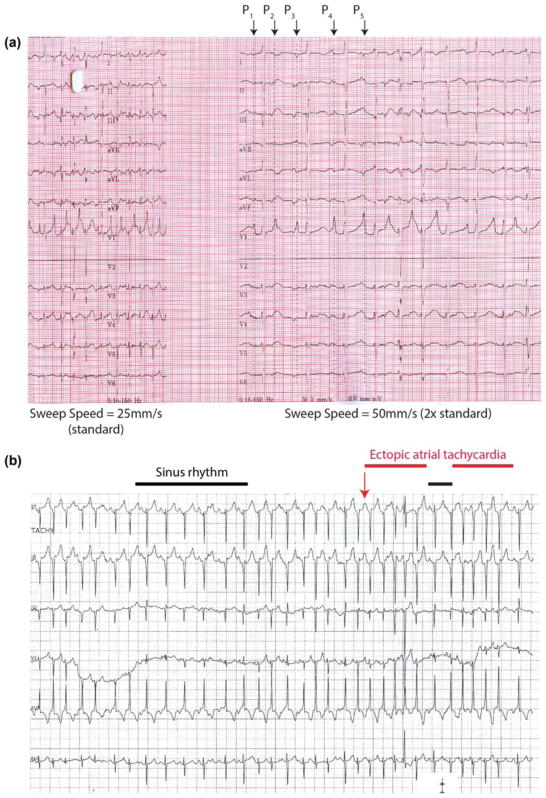
Figure 2 shows representative rhythm strips from a patient in MAT (Patient 3, Figure 2a) and EAT in a different patient (Patient 8, Figure 2b). Identification of multiple P-wave morphologies was facilitated by recording the trace at twice the normal sweep speed (50 mm/s; Figure 2a). By contrast, EAT tracings demonstrate typical findings of two different P-wave morphologies (one representing sinus rhythm) with irregular RR intervals (Figure 2b). In contrast to reentrant supraventricular tachycardia which demonstrates abrupt initiation and termination of the abnormal rhythm, intermittent sinus beats can be seen interspersed between tachycardia runs in Figure 2b, which is characteristic of EAT.
FIGURE 2.
Rhythm strips showing multi-focal atrial tachycardia and ectopic atrial tachycardia (a) 12 lead rhythm strip from Patient 3 recorded at sweep speeds of both 25 and 50 mm/s demonstrating multifocal atrial tachycardia with at least five different P-wave morphologies (labeled P1 to P5). Note how the increased sweep speed (50 mm/s) facilitates diagnosis when compared to the portion recorded at the standard sweep speed (25 mm/s). (b) Rhythm strip from patient 8 demonstrating episodes of sinus rhythm (denoted by black bar) followed by intermittent episodes of ectopic atrial tachycardia (red bar). In contrast to the tracing from (a), P-wave morphology, axis and amplitude are roughly the same. Unlike reentrant arrhythmias, patients may quickly enter and exit atrial tachycardia and have sinus beats interspersed between nonsinus beats as seen here. [Color figure can be viewed at wileyonlinelibrary.com]
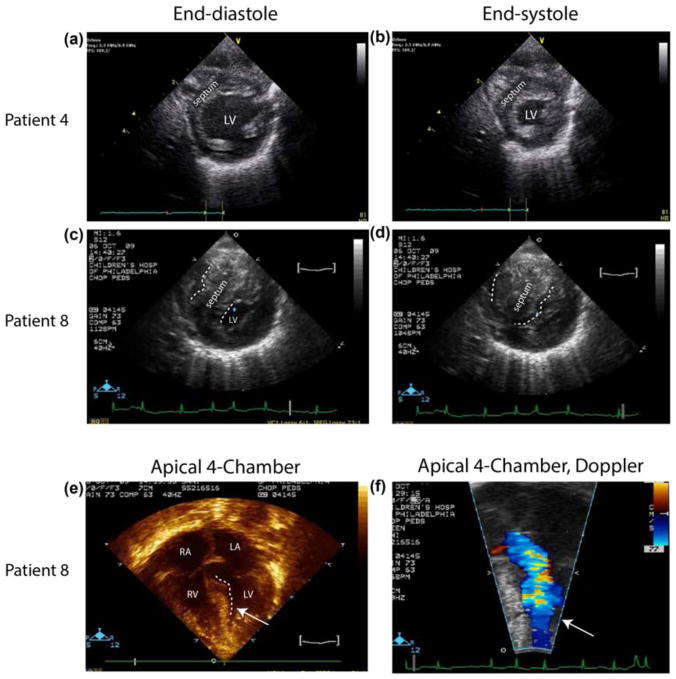
Each patient had an echocardiogram at the presentation of arrhythmia. Signs of cardiomyopathy were present in seven patients, while four patients showed no overt evidence of HCM. HCM is typically defined by abnormal wall thickness (Elliott & McKenna, 2004; Maron, 2006). Figure 3 provides parasternal short axis images at end-diastole and end-systole for one patient showing tachycardia without evidence of cardiomyopathy (Figure 3a,b, Patient 4) as well as images from one patient who had both atrial tachycardia and hypertrophic cardiomyopathy (Figure 3c–f, Patient 8). Echocardiographic images from Patient 4 show normal septal (labeled) and posterior left ventricular free wall thickness (Figure 3a,b). By contrast, short axis images from Patient 8 show massive septal hypertrophy that nearly obliterates the left ventricular cavity at end systole (Figure 3d). Note that during ventricular filling (end-diastole, Figure 3c) the left ventricular volume available for filling is greatly reduced compared to the filling area of the nonhypertrophic heart (Figure 3a). Four chamber imaging of Patient 8 demonstrates the ventricular septum protruding into the left ventricular outflow tract (Figure 3e,f) and companion Doppler flow imaging shows mid left ventricular cavity flow acceleration which defines a subset hypertrophic cardiomyopathy.
FIGURE 3.
Parasternal short axis echocardiographic images from RASopathy patients presenting with nonreentrant atrial tachycardia showing ventricular wall dimensions at both end-diastole (a, c), and end-systole (b, d), for patient 4 (a, b), patient 8 (c–f). Note that images from patient 4 show normal septal and free wall thickness, while those images from patient 8 show hypertrophied septum and myocardium, with muscle nearly obliterating the LV cavity at end systole in patient 8. Four chamber imaging from patient 8 demonstrates left ventricular outflow tract obstruction (e) and Doppler flow imaging of the identical region shows mid-cavitary flow acceleration (f). [Color figure can be viewed at wileyonlinelibrary.com] prominently in the clinical presentation, a number of distinct phenotypic features prompted testing for RAS/MAPK disorders (Table 1). Postnatally, hyper-pigmented macules and dysmorphic craniofacial features prompted molecular testing with increased suspicion for RASopathy. Additional features suggesting RASopathies included down slanted palpebral fissures, wide nasal root with flat bridge, low set dysmorphic ears, and coarse facial features.
Quantitative echocardiographic data were available for eleven patients (Table 1) including LVeDD (z-score range: −3.84 to 2.77), LVeSD (z-score range: −5.02 to 5.32), and LV shortening fraction (range: 38.9 to 66%). Left ventricular mass z-score was available for 10 of 11 patients and ranged from −3.27 to +9.42. No patient had complex congenital heart disease, but septal defects were detected in three patients. In addition, six patients demonstrated valvar pulmonary stenosis, which was severe in one patient. No other hemodynamically significant congenital heart defects were diagnosed.
3.4 | Arrhythmia treatment
Patients presenting in the neonatal period often had mild hemodynamic compromise and poor feeding over time prior to restoration of sinus rhythm. Initial medical therapies included propranolol in 10 patients. Treatment with propranolol alone and/or with digoxin failed to maintain sinus rhythm in all but one patient (Table 1). Flecainide alone or in combination with propranolol was the most common chronic anti-arrhythmic treatment chosen. Amiodarone alone was used successfully for two patients, although one patient subsequently transitioned to propranolol and verapamil due to amiodarone toxicity. Anti-arrhythmic agents were successfully weaned in all patients, except for one who died from complications of a rhabdomyosarcoma and a second who underwent cardiac transplantation, in part because of functional hemodynamic instability that was compounded by rhythm disturbance despite flecainide treatment. In the 10 patients whose clinical arrhythmias resolved, medications were discontinued at a mean age of 28.0 months (range 6.5–49 months). A third child from our cohort died from complications of cardiac surgery (septal myomectomy). This child’s arrhythmia resolved prior to surgery, so this case was not considered an anti-arrhythmic treatment failure. A 4-month-old child with cellulitis apparently died of complications of systemic infection, unrelated to arrhythmia.
4 | DISCUSSION
This multi-institutional case series illustrates an association between mutations in RAS/MAPK signaling pathway genes and nonreentrant atrial tachycardia (MAT and EAT). Importantly, rhythm disturbances occurred both in the presence and absence of cardiac hypertrophy. We observed predominantly nonreentrant atrial tachycardia in our cohort (i.e., EAT and MAT, which is also known as CAT). Reportedly, 84% of atrial arrhythmias in children arise from reentrant circuits involving accessory pathways (Ko, JK, BJ, Strasburger, and Benson DW jr., 1992). In contrast, in our cohort, we observed nonreentrant atrial arrhythmias, which had previously been identified only in Costello syndrome patients (Lin et al., 2011).
Distinguishing between reentrant and nonreentrant arrhythmias can be critically important as therapies and prognoses differ greatly between these two entities. Propranolol and digoxin continue to be the preferred first line treatment for reentrant arrhythmias. However, we found that infants with RASopathies and atrial tachycardia can be difficult to manage, partially because propranolol and digoxin fail to convert or maintain sinus rhythm in patients with MAT and EAT. By contrast, in this cohort, effective medications included flecainide alone, flecainide in combination with propranolol, or amiodarone alone. While propranolol can be used to treat EAT effectively in other patient populations, it is not immediately clear why this drug is less effective in this subgroup of patients. Importantly, arrhythmia resolved or was well-controlled in all but two cases. The former were successfully weaned off anti-arrhythmic medications after a period ranging from 6.5 to 49 months. The latter group included a 4 months old boy who was found pulseless and unresponsive during inpatient cellulitis treatment and died from an unclear etiology and another patient who underwent cardiac transplantation. This second patient had poorly tolerated break-through tachycardia in the context of worsening left ventricular outflow obstruction. While an atrial arrhythmia episode would not be predicted to cause catastrophic hemodynamic compromise, we cannot exclude the possibility that atrial arrhythmia complicated this boy’s death.
While flecainide, a sodium channel blocker, has been used successfully to treat ectopic or MAT for over 20 years (Houyel, Fournier, & Davignon, 1990), the mechanism of its efficacy is not immediately obvious and remains controversial (Bannister et al., 2015; Hilliard et al., 2010; Smith & Macquaide, 2015; Watanabe et al., 2009). Previous studies suggested that MAT and EAT result from disrupted calcium homeostasis leading to triggered activity (Levine, Michael, & Guarnieri, 1985; Marchlinski & Miller, 1985). Flecainide, by contrast, is traditionally classified as a sodium channel blocker (Vaughan Williams, 1975), and sodium channel blockers would not be expected to affect calcium homeostasis directly. Importantly, it has been argued that flecainide diminishes calcium release from the sarcoplasmic reticulum through ryanodine receptors in addition to its direct inhibitory action on sodium channels (Bannister et al., 2015; Mehra, Imtiaz, van Helden, Knollmann, & Laver, 2014; Smith & Macquaide, 2015; Watanabe et al., 2009). Again, nonreentrant arrhythmias arise secondary to dysregulated calcium release, such as inappropriate calcium release from the sarcoplasmic reticulum through the ryanodine receptor. While it is difficult to imagine how direct sodium channel blockade would diminish sarcoplasmic reticulum calcium release and treat nonreentrant arrhythmias, perhaps flecainide’s direct or indirect action on the ryanodine receptor accounts for the drug’s efficacy in treating MAT and EAT. The fact that flecainide, a drug used to restore calcium homeostasis, is an effective anti-arrhythmic for MAT and EAT in RASopathy patients suggests that disordered calcium release may result directly from RASopathy mutation expression.
In addition to increased arrhythmogenesis, disordered calcium handling can contribute to cardiomyopathy and heart failure (Anastasaki, Estep, Marais, Rauen, & Patton, 2009; Lan et al., 2013). Incessant atrial arrhythmias may cause cardiomyopathy and diminished cardiovascular function. Conversely, arrhythmias may be secondary to the pressure load conferred by a cardiomyopathy. Finally, the presence of cardiomyopathy prior to arrhythmia might suggest that nonreentrant atrial tachycardia is secondary to elevated end-diastolic pressure subsequent to cardiomyopathy (pressure load). In this retrospective case series, we found that 4 (36%) of 11 patients presenting with nonreentrant atrial tachycardia had no evidence of cardiomyopathy as indicated by increased wall thickness. Thus, at least some atrial tachycardia in our cohort did not arise secondary to pressure overload, again suggesting disordered calcium release arises as a result of some other mechanism.
Hypertrophic cardiomyopathy is defined by abnormal wall thickness (Elliott & McKenna, 2004; Maron, 2006; McNally, Barefield, & Puckelwartz, 2015). Seven (64%) of our 11 patients demonstrated cardiac hypertrophy, meeting American Heart Association criteria for hypertrophic cardiomyopathy. Four of the seven with hypertrophy demonstrated asymmetric septal hypertrophy. While 4 of 11 (36%) patients did not meet traditional criteria for diagnosing hypertrophic cardiomyopathy, despite repeated episodes of atrial tachycardia, they had elevated shortening fractions (Patient 1: 39%; Patient 4: 42%; Patient 7: 56%; Patient 10: 46%). This increased shortening fraction suggests that these patients have elevated intracellular calcium concentrations since there is a strong dependence between intracellular calcium concentration and myocyte contraction force (Kentish, ter Keurs, Ricciardi, Bucx, & Noble, 1986; Sun & Irving, 2010).
Thus, several observations suggest that RASopathy patients exhibit calcium dysregulation that can give rise to cardiomyopathy and/or atrial tachycardia. First, RASopathy patients demonstrate MAT and EAT thought to be initiated by triggered activity (Bradley et al., 2001; Marchlinski & Miller, 1985; McCord & Borzak, 1998). Furthermore, triggered activity is caused by disordered calcium homeostasis, that frequently results from inappropriate calcium release from ryanodine receptors (Wehrens et al., 2004). Medications diminishing ryanodine receptor calcium release, such as flecainide, have been particularly effective in treating RASopathy patients with MAT or EAT, suggesting that restoring calcium homeostasis by decreasing calcium release results in decreased triggered activity and diminished arrhythmia. Finally, increased shortening fraction is consistent with increased calcium release from intracellular stores and those RASopathy patients not showing overt cardiomyopathy demonstrate hyper-contractile shortening fractions. These observations may provide the basis for testable hypotheses directed at understanding RASopathy cardiac pathology. Such testing could include expression of RASopathy-causing mutations in animal models or patient derived induced pluripotent stem cells (iPSC) and quantifying intracellular calcium content or numbers of calcium release events in models versus controls.
The current study is limited by its small patient numbers. Given the relatively rare occurrence of non-Noonan syndrome RASopathies, we pooled patients from a multi-institutional network. Although Cardiofaciocutaneous syndrome was not represented in this series, it is likely that nonreentrant tachycardia will be observed. Further work to verify our observations in a larger cohort should be performed, ideally in a prospective manner. Additional studies using animal models or patient-specific expression systems should be used to test the hypotheses raised here. This would be an important first step in deepening our understanding of RASopathy cardiac disease mechanisms and could ultimately lead to the identification and development of novel therapeutics for patients with RASopathies.
In conclusion, we report that patients with RAS/MAPK pathway mutations have nonreentrant atrial tachycardia that is poorly treated by propranolol or digoxin (typical first-line SVT treatments), but responds well to flecainide or amiodarone alone or in concert with propranolol. Atrial tachycardia in RASopathy patients occurred in the presence and absence of hypertrophic cardiomyopathy. Those patients with nonreentrant tachycardia without hypertrophic cardiomyopathy frequently demonstrated hyper-dynamic function as evidenced by increased shortening fraction, suggesting that they possessed increased intracellular calcium. Nonreentrant tachycardia resolved in 9 of 11 patients. In addition to providing insights that will aid management of patients with a RASopathy, the clinical findings here suggest the hypothesis that activating RAS/ MAPK pathway mutations result in intracellular calcium dysregulation that leads to either arrhythmia, cardiac hypertrophy, or both. There is a need for additional investigation and longitudinal study in this patient population to define evidence based guidelines for cardiac surveillance, but, in the interim, contributing authors have suggested collaborative expert opinion consensus statements may also be useful. Toward this end, we would suggest raising suspicion for arrhythmia and cardiomyopathy in neonatal RASopathy patients. Respiratory distress, diminished feeding tolerance, or unexplained irritability should suggest cardiac evaluation, including ECG, echocardiogram and 24 hr ambulatory ECG monitoring. In addition to typical signs and symptoms of arrhythmia or cardiomyopathy, evidence of hyperdynamic cardiac function seen on echocardiogram should also raise suspicion for arrhythmia, cardiomyopathy or nascent cardiomyopathy as this was present in some study patients with arrhythmia prior to overt chamber dimension abnormalities. This case series should increase awareness among pediatric cardiologists and geneticists about these increasingly recognized syndromes and provide clinical information assisting the diagnosis and management of cardiac disease, and, ultimately promote understanding of the molecular mechanisms underlying these diseases.
Acknowledgments
Funding information: NIH, Grant/Award Number: K08 HL94748
The authors wish to thank their patients and families for their help and cooperation in compiling this manuscript. The authors would also like to acknowledge Dr. Amy Roberts and Dr. Terrence Prendeville from Boston Children’s Hospital who queried their Noonan Syndrome patient registry and communicated their observations related to atrial arrhythmias in their patient cohort. This work was supported by NIH grant K08 HL94748 to MDL. Dr. Lin and Dr. Wenger are Associate Editors of the American Journal of Medical Genetics.
References
- Anastasaki C, Estep AL, Marais R, Rauen KA, Patton EE. Kinase-activating and kinase-impaired cardio-facio-cutaneous syndrome alleles have activity during zebrafish development and are sensitive to small molecule inhibitors. Human Molecular Genetics. 2009;18(14):2543–2554. doi: 10.1093/hmg/ddp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister ML, Thomas NL, Sikkel MB, Mukherjee S, Maxwell C, MacLeod KT, … Williams AJ. The mechanism of flecainide action in CPVT does not involve a direct effect on RyR2. Circulation Research. 2015;116(8):1324–1335. doi: 10.1161/CIRCRESAHA.116.305347. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Fischbach PS, Law IH, Serwer GA, Dick IIM. The clinical course of multifocal atrial tachycardia in infants and children. Journal of the American College of Cardiology. 2001;38:401–408. doi: 10.1016/s0735-1097(01)01390-0. [DOI] [PubMed] [Google Scholar]
- Dodo H, Gow RM, Hamilton RM, Freedom RM. Chaotic atrial rhythm in children. American Heart Journal. 1995;129(5):990–995. doi: 10.1016/0002-8703(95)90121-3. [DOI] [PubMed] [Google Scholar]
- Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet (London, England) 2004;363(9424):1881–1891. doi: 10.1016/S0140-6736(04)16358-7. [DOI] [PubMed] [Google Scholar]
- Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, … Knollmann BC. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. Journal of Molecular and Cellular Cardiology. 2010;48(2):293–301. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houyel L, Fournier A, Davignon A. Successful treatment of chaotic atrial tachycardia with oral flecainide. International Journal of Cardiology. 1990;27(1):27–29. doi: 10.1016/0167-5273(90)90187-a. [DOI] [PubMed] [Google Scholar]
- Ko JK, Deal BJ, Strasburger JF, Benson DW., Jr Supra-ventricular tachycardia mechanisms and their age distribution in pediatric patients. The American Journal of Cardiology. 1992;69(12):1028–1032. doi: 10.1016/0002-9149(92)90858-v. [DOI] [PubMed] [Google Scholar]
- Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Circulation Research. 1986;58(6):755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, … Wu JC. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12(1):101–113. doi: 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JH, Michael JR, Guarnieri T. Treatment of multifocal atrial tachycardia with verapamil. The New England Journal of Medicine. 1985;312(1):21–25. doi: 10.1056/NEJM198501033120105. [DOI] [PubMed] [Google Scholar]
- Lin AE, Alexander ME, Colan SD, Kerr B, Rauen KA, Noonan J, … Gripp KW. Clinical, pathological, and molecular analyses of cardiovascular abnormalities in Costello syndrome: A Ras/MAPK pathway syndrome. American Journal of Medical Genetics Part A. 2011;155(3):486–507. doi: 10.1002/ajmg.a.33857. [DOI] [PubMed] [Google Scholar]
- Marchlinski FE, Miller JM. Atrial arrhythmias exacerbated by theophylline. Response to verapamil and evidence for triggered activity in man. CHEST Journal. 1985;88(6):931–934. doi: 10.1378/chest.88.6.931. [DOI] [PubMed] [Google Scholar]
- Maron BJ. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement From the Council on Clinical Cardiology, Heart Failure and Transplantation Committee. Circulation. 2006;113(14):1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- McCord J, Borzak S. Multifocal atrial tachycardia. CHEST Journal. 1998;113(1):203–209. doi: 10.1378/chest.113.1.203. [DOI] [PubMed] [Google Scholar]
- McNally EM, Barefield DY, Puckelwartz MJ. The Genetic landscape of cardiomyopathy and its role in heart failure. Cell Metabolism. 2015;21(2):174–182. doi: 10.1016/j.cmet.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra D, Imtiaz MS, van Helden DF, Knollmann BC, Laver DR. Multiple modes of ryanodine receptor 2 inhibition by flecainide. Molecular Pharmacology. 2014;86(6):696–706. doi: 10.1124/mol.114.094623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, … Gelb BD. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nature Genetics. 2007;39(8):1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- Pierpont MEM, Magoulas PL, Adi S, Kavamura MI, Neri G, Noonan J, … Rauen KA. Cardio-facio-cutaneous syndrome: Clinical features, diagnosis, and management guidelines. Pediatrics. 2014;134(4):e1149–e1162. doi: 10.1542/peds.2013-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendiville TW, Gauvreau K, Tworog-Dube E, Patkin L, Kucherlapati RS, Roberts AE, Lacro RV. Cardiovascular disease in Noonan syndrome. Archives of Disease in Childhood. 2014;99(7):629–634. doi: 10.1136/archdischild-2013-305047. [DOI] [PubMed] [Google Scholar]
- Rauen KA. The RASopathies. Annual Review of Genomics and Human Genetics. 2013;14:355–369. doi: 10.1146/annurev-genom-091212-153523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen KA, Tidyman WE, Estep AL, Sampath S, Peltier HM, Bale SJ, Lacassie Y. Molecular and functional analysis of a novel MEK2 mutation in cardio-facio-cutaneous syndrome: Transmission through four generations. American Journal of Medical Genetics Part A. 2010;152A(4):807–814. doi: 10.1002/ajmg.a.33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Tetsu O, Tidyman WE, Estep AL, Conger BA, Cruz MS, … Rauen KA. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311(5765):1287–1290. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- Salerno DM, Anderson B, Sharkey PJ, Iber C. Intravenous verapamil for treatment of multifocal atrial tachycardia with and without calcium pretreatment. Annals of Internal Medicine. 1987;107(5):623–628. doi: 10.7326/0003-4819-107-5-623. [DOI] [PubMed] [Google Scholar]
- Smith GL, Macquaide N. The direct actions of flecainide on the human cardiac ryanodine receptor: Keeping open the debate on the mechanism of action of local anesthetics in CPVT. Circulation Research. 2015;116(8):1284–1286. doi: 10.1161/CIRCRESAHA.115.306298. [DOI] [PubMed] [Google Scholar]
- Sun YB, Irving M. The molecular basis of the steep force-calcium relation in heart muscle. Journal of Molecular and Cellular Cardiology. 2010;48(5):859–865. doi: 10.1016/j.yjmcc.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Cotter PD, Zampino G, Gelb BD, Rauen KA. Exclusion of PTPN11 mutations in Costello syndrome: Further evidence for distinct genetic etiologies for Noonan, cardio-facio-cutaneous and Costello syndromes. Clinical Genetics. 2003;63(5):423–426. doi: 10.1034/j.1399-0004.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- Theis JL, Zimmermann MT, Larsen BT, Rybakova IN, Long PA, Evans JM, … Olson TM. TNNI3K mutation in familial syndrome of conduction system disease, atrial tachyarrhythmia and dilated cardiomyopathy. Human Molecular Genetics. 2014;23(21):5793–5804. doi: 10.1093/hmg/ddu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidyman WE, Rauen KA. The RASopathies: Developmental syndromes of Ras/MAPK pathway dysregulation. Current Opinion in Genetics & Development. 2009;19(3):230–236. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. Journal of Clinical Investigation. 2013;123(1):37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan Williams EM. Classification of antidysrhythmic drugs. Pharmacology & Therapeutics. Part B: General & Systematic Pharmacology. 1975;1(1):115–138. doi: 10.1016/0306-039x(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, … Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nature Medicine. 2009;15(4):380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XHT, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, … Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science (New York, NY) 2004;304(5668):292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- Wu X, Simpson J, Hong JH, Kim KH, Thavarajah NK, Backx PH, … Araki T. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1(L613V) mutation. Journal of Clinical Investigation. 2011;121(3):1009–1025. doi: 10.1172/JCI44929. [DOI] [PMC free article] [PubMed] [Google Scholar]