Central nervous system (CNS) relapse of systemic diffuse large B-cell lymphoma (DLBCL) is an uncommon, devastating complication of this disease (Kridel and Dietrich 2011). The addition of rituximab to anthracycline-based chemotherapy (immunochemotherapy, IC) has improved outcomes in patients with DLBCL(Coiffier, et al 2010), with reported CNS relapse rates of 2–9.7% depending on the study population and duration of follow-up(Kumar, et al 2012, Villa, et al 2010). CNS relapses in systemic DLBCL typically occur within 4.7–8 months after diagnosis (Bernstein, et al 2009, Boehme, et al 2007, Villa, et al 2010), suggesting a component of occult CNS disease at diagnosis or primary chemotherapy refractory CNS disease. Late CNS relapse is infrequent.
Combinations of targeted agents with frontline IC (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone + other agent; R[X]-CHOP) are increasingly being assessed to improve outcomes in DLBCL(Nowakowski, et al 2015, Younes, et al 2014). It is unclear to what degree these influence the incidence of CNS relapse in DLBCL because agents such as lenalidomide and ibrutinib penetrate the blood-brain barrier(Bernard, et al 2015). The event-free survival at 24 months (EFS24) is a validated endpoint for disease-related outcome in DLBCL in the IC era(Maurer, et al 2014). Establishing that the incidence and pattern of CNS relapse also follows the EFS24 timeframe would be valuable. EFS24 would therefore serve as an informative endpoint specifically in the comparison of different regimens’ potential to alter the incidence of CNS relapse.
Here we present the incidence and define the timing of CNS relapse in 1017 patients with DLBCL treated with IC and establish that EFS24 is an appropriate endpoint for evaluating the risk of CNS relapse. After obtaining approval of the human subject institutional review board at the Mayo Clinic and University of Iowa, newly diagnosed human immunodeficiency virus-negative patients aged ≥18 years with DLBCL or primary mediastinal B-cell lymphoma (PMBCL) treated with anthracycline-based IC were prospectively enrolled within 9 months of diagnosis on the Molecular Epidemiology Resource (MER) of University of Iowa / Mayo Clinic Specialized Program of Research Excellence (SPORE) between 2002 and 2012(Maurer, et al 2014). Patients with CNS involvement at diagnosis, primary CNS lymphoma, or post-transplant lymphoproliferative disorder at diagnosis were excluded.
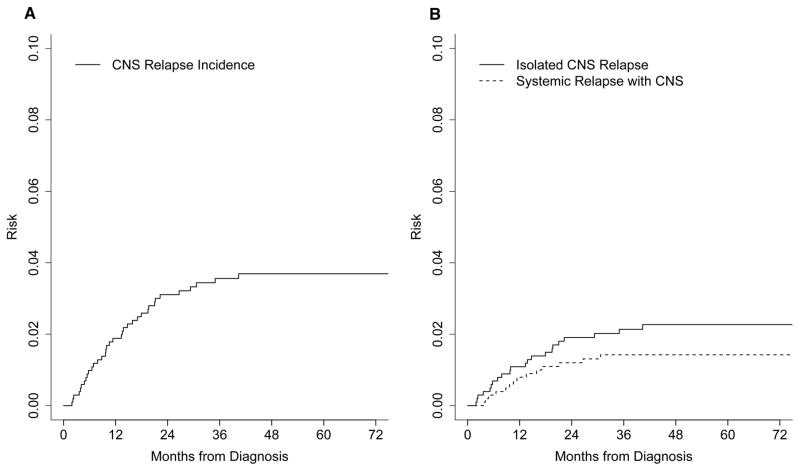
Baseline characteristics and frontline regimens are summarized in Table I. At a median follow-up of 59 months (1– 148), 404 patients (40%) had an outcome event (relapse, retreatment or death) and 295 patients (29%) had died. Thirty-six of the 1017 (3.5%) patients had a relapse involving the CNS; 22 had isolated CNS relapse and 14 had combined CNS and systemic relapse. The location of CNS relapse was parenchymal in 22 patients, leptomeningeal in 11 patients and both parenchymal and leptomeningeal in 3 patients. The cumulative incidence of CNS relapse was 3.1% (95% confidence interval [CI] 2.2% – 4.4%) at 2 years and 3.7% (95% CI 2.7–5.0%) at 5 years (Figure 1A). Isolated CNS relapse without concurrent systemic relapse had an incidence of 1.9% (95% CI 1.2%–3.0%) at 2 years of diagnosis (Figure 1B). CNS relapse occurred after first line IC in 25 patients and after a salvage regimen in 11 patients.
Table I.
Characteristics at diagnosis of patients with and without subsequent CNS relapse
| Patients with CNS relapse (n = 36) | Patients without CNS relapse (n = 981) | P-value | |
|---|---|---|---|
|
| |||
| Age at diagnosis, years; mean (range) | 61 (20–86) | 62 (18–92) | 0.87 |
|
| |||
| Male | 25 (69%) | 542 (55%) | 0.092 |
|
| |||
| IPI | 0.17 | ||
| 0–1 | 6 (17%) | 339 (35%) | |
| 2 | 13 (36%) | 286 (29%) | |
| 3 | 11 (31%) | 231 (24%) | |
| 4–5 | 6 (17%) | 125 (13%) | |
|
| |||
| Initial therapy | 0.23 | ||
| R-CHOP | 26 (72%) | 803 (83%) | |
| R-EPOCH | 5 (14%) | 60 (6%) | |
| R2-CHOP | 3 (8%) | 41 (4%) | |
| ER-CHOP | 1 (3%) | 22 (2%) | |
| Other | 1 (3%) | 55 (6%) | |
|
| |||
| Cell of origin (available in 17) | |||
| GCB | 8 (47%) | 199 (36%) | 0.15 |
| Non-GCB | 9 (54%) | 355 (64%) | |
|
| |||
| Extranodal sites of disease | |||
| Testicular | 2 (6%) | 14 (1%) | 0.051 |
| Renal/adrenal | 5 (14%) | 31 (3%) | 0.0006 |
| Bone | 10 (28%) | 183 (19%) | 0.17 |
| Bone marrow involvement | 0.18 | ||
| Low grade | 3 (8%) | 44 (4%) | |
| DLBCL | 6 (17%) | 94 (10%) | |
| None | 27 (75%) | 843 (86%) | |
| No extranodal involvement | 7 (19%) | 612 (63%) | <0.0001 |
|
| |||
| Elevated LDH | 22 (73%) | 512 (58%) | 0.14 |
|
| |||
| CNS-IPI | 0.060 | ||
| Low risk | 6 (17%) | 339 (34%) | |
| Intermediate risk | 22 (66%) | 503 (52%) | |
| High risk | 8 (17%) | 136 (14%) | |
CNS: central nervous system; DPBCL: diffuse large B cell lymphoma; ER-CHOP: R-CHOP + epratuzumab; GCB: germinal centre B cell; IPI: International Prognostic Index; LDH: lactate dehydrogenase; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-EPOCH: rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; R2-CHOP: R-CHOP + lenalidomide.
Figure 1.
A. Cumulative incidence of all central nervous system (CNS) relapse. B. Cumulative incidence of isolated CNS relapse (solid line) versus CNS relapse occurring with systemic relapse (dashed line).
Seventeen of the 36 patients with CNS relapse (47%) had an International Prognostic Index (IPI) ≥3. Lactate dehydrogenase was elevated in 22 (73%) patients. CNS-IPI Risk Score was intermediate or high risk in 30 patients (83%). Cell of origin per Hans algorithm was available in 17 patients, 9 patients of which were non-germinal centre B cell (54%). Extranodal sites of disease among these 36 patients were testicular (2), renal (5), bone (10) and bone marrow (6). Notably, 7 patients (19%) had no extranodal involvement. Among patients with CNS relapse, 6 patients had received CNS prophylactic therapy as part of their frontline regimen. Two patients received 3 cycles of intermediate-dose [3.5 g/m2], one patient received 2 cycles of intermediate-dose methotrexate, 1 patient received 4 doses of intrathecal methotrexate and 1 patient received the intrathecal methotrexate and cytarabine part the R-EPOCH regimen (rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin). Three of these patients subsequently developed isolated relapse in the CNS.
The median overall survival after CNS relapse was 6.3 months (95% CI: 2.9–15.5), with 29% (95% CI: 17%–49%) remaining alive at 12 months. The median overall survival in patients who developed CNS relapse after frontline IC was 7.6 months versus 2.1 months in patients who developed CNS relapse after salvage chemotherapy (p = 0.005). First line therapy after CNS relapse included high dose methotrexate-based chemotherapy in 22 patients, other systemic regimens in 6 patients (4 RICE [rituximab, ifosfamide, cyclophosphamide and etoposide], 1 temozolomide and rituximab, and 1 R-hyperCVAD [cyclophosphamide, vincristine, doxorubicin and dexamethasone) and intrathecal methotrexate and/or cytarabine in 5 patients.
Ten patients (28%) proceeded to autologous stem cell transplant after CNS relapse, 6 remain alive at a median follow-up of 22 months (range 2–93). There were no differences in post-CNS relapse survival by age, sex, diagnostic CNS-IPI, cell of origin, time of CNS relapse from diagnosis, diagnostic extranodal site involvement or site of CNS relapse (results not shown, all p >0.20)
In this large, prospective cohort with a median overall follow-up of over 5 years, we identified that most patients who develop CNS relapse will do so within the first 2 years. These data demonstrate that the timeframe of CNS relapse is consistent with the standard for disease-related outcome in DLBCL, the EFS24. Our study affirms that EFS24 captures the timeframe of most CNS relapses and therefore represents a powerful endpoint for prospective trials assessing combinations of R-CHOP with novel agents (R[X]-CHOP) that demonstrate CNS penetration. Given the relative infrequency of CNS relapse of DLBCL, prospective, randomized trials would require a prohibitively large sample size to detect a change in a fairly low incidence rate. Therefore, a method of comparing incidence data in a standardized manner across earlier phase studies is required. EFS24 in this setting can provide consensus evaluation of the influence of these newer regimens on the risk of CNS relapse. Given the timeframe of CNS relapse, as established in this study, the EFS24 would also be valuable in retrospective and prospective studies assessing optimal CNS prophylactic strategies. Capturing the vast majority of events in a relatively short time frame, the EFS24 is a clinically relevant endpoint for CNS relapse of DLBCL.
Acknowledgments
GT, MJM, TEW and GSN designed the research study. GT, MJM and UF performed the research and clinical data extraction. GT, MJM, UF, TEW and GSN analysed the data. GT, MJM, UF, TEW, GSN wrote the paper. CAT, NNB, SMA, LFA, WRM, SIS, JRC, TMH and BKL contributed to the acquisition and interpretation of data and revised the paper critically. All authors approved the submitted version.
Funding
This research was supported by the Mayo Clinic / University of Iowa Lymphoma SPORE grant from the National Institute of Health (P50 CA97274-13).
References
- Bernard S, Goldwirt L, Amorim S, Brice P, Brière J, de Kerviler E, Mourah S, Sauvageon H, Thieblemont C. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood. 2015;126:1695–1698. doi: 10.1182/blood-2015-05-647834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein SH, Unger JM, LeBlanc M, Friedberg J, Miller TP, Fisher RI. Natural History of CNS Relapse in Patients With Aggressive Non-Hodgkin’s Lymphoma: A 20-Year Follow-Up Analysis of SWOG 8516—The Southwest Oncology Group. Journal of Clinical Oncology. 2009;27:114–119. doi: 10.1200/JCO.2008.16.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme V, Zeynalova S, Kloess M, Loeffler M, Kaiser U, Pfreundschuh M, Schmitz N. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007;18:149–157. doi: 10.1093/annonc/mdl327. [DOI] [PubMed] [Google Scholar]
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M, Sebban C, Belhadj K, Bordessoule D, Ferme C, Tilly H. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kridel R, Dietrich PY. Prevention of CNS relapse in diffuse large B-cell lymphoma. The Lancet. Oncology. 2011;12:1258–1266. doi: 10.1016/S1470-2045(11)70140-1. [DOI] [PubMed] [Google Scholar]
- Kumar A, Vanderplas A, LaCasce AS, Rodriguez MA, Crosby AL, Lepisto E, Czuczman MS, Nademanee A, Niland J, Gordon LI, Millenson M, Zelenetz AD, Friedberg JW, Abel GA. Lack of benefit of central nervous system prophylaxis for diffuse large B-cell lymphoma in the rituximab era: findings from a large national database. Cancer. 2012;118:2944–2951. doi: 10.1002/cncr.26588. [DOI] [PubMed] [Google Scholar]
- Maurer MJ, Ghesquieres H, Jais JP, Witzig TE, Haioun C, Thompson CA, Delarue R, Micallef IN, Peyrade F, Macon WR, Jo Molina T, Ketterer N, Syrbu SI, Fitoussi O, Kurtin PJ, Allmer C, Nicolas-Virelizier E, Slager SL, Habermann TM, Link BK, Salles G, Tilly H, Cerhan JR. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:1066–1073. doi: 10.1200/JCO.2013.51.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski GS, LaPlant B, Macon WR, Reeder CB, Foran JM, Nelson GD, Thompson CA, Rivera CE, Inwards DJ, Micallef IN, Johnston PB, Porrata LF, Ansell SM, Gascoyne RD, Habermann TM, Witzig TE. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:251–257. doi: 10.1200/JCO.2014.55.5714. [DOI] [PubMed] [Google Scholar]
- Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21:1046–1052. doi: 10.1093/annonc/mdp432. [DOI] [PubMed] [Google Scholar]
- Younes A, Thieblemont C, Morschhauser F, Flinn I, Friedberg JW, Amorim S, Hivert B, Westin J, Vermeulen J, Bandyopadhyay N, de Vries R, Balasubramanian S, Hellemans P, Smit JW, Fourneau N, Oki Y. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. The Lancet. Oncology. 2014;15:1019–1026. doi: 10.1016/S1470-2045(14)70311-0. [DOI] [PubMed] [Google Scholar]