Abstract
Peptides and peptidomimetics are emerging as an important class of clinic therapeutics. Here we report a peptide-guided method for the functionalization and macrocyclization of bioactive peptidosulfonamides by Pd(II)-catalyzed late-stage C–H activation. In this protocol, peptides act as internal directing groups and enable site-selective olefination of benzylsulfonamides and cyclization of benzosulfonamides to yield benzosultam-peptidomimetics. Our results provide an unusual example of benzosulfonamide cyclization with olefins through a sequential C–H activation, which involves the generation of a reactive palladium-peptide complex. Furthermore, this protocol allows facile self-guided macrocyclization of sulfonamide-containing peptides by intramolecular olefination with acrylates and unactivated alkenes, affording bioactive peptidosulfonamide macrocycles of various sizes. Together, our results highlight the utility of peptides as internal directing groups in facilitating transition metal-catalyzed functionalization of peptidomimetics.
Aryl sulfonamides and sultams are important pharmacophores in medicinal chemistry. Here, the authors report a practical palladium-catalyzed C–H activation assisted by amino-acid residues in the substrate leading to arylsulfonamides and bioactive peptidosulfonamide macrocycles.
Introduction
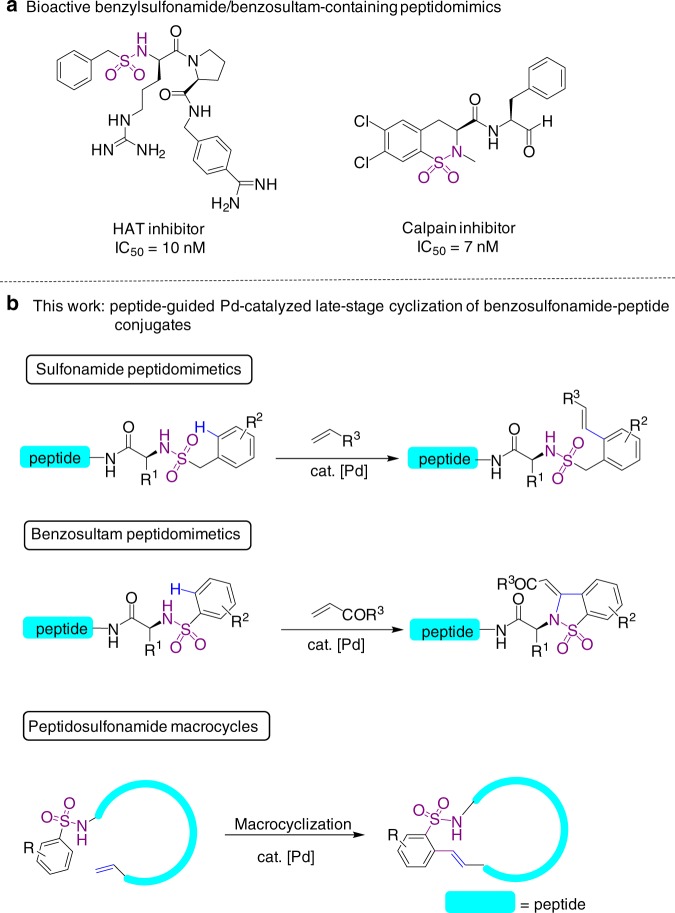
Peptides and peptidomimetics are emerging as clinic theraputics with high potency and selectivity.1,2 One major driving force for the growing interest of these compounds is their capability in regulating protein–protein interactions, which have been identified in numerous disease-related biologica process. Therefore, it is highly desirable to develop chemical strategies to synthesize bioactive peptides and peptidomimetics with structural diversity. For example, arylsulfonamides and sultams (cyclic sulfonamide) are important pharmacophores in medicinal chemistry,3–7 and introduction of sulfonamide functionality into peptides usually provides improved proteolytic stability, hydrogen-bonding possibilities and improved biological activities.8–11 Specifically, peptidomimetics containing benzofused sulfonamides or sultams are among the most potent inhibitors of disease-related proteases, and benzosultam derivatives often exhibit improved pharmaceutical properties (Fig. 1a).12–15 Despite their promising bioactivities, the development of this class of compounds is hindered by the lack of facile synthetic methodologies, especially for the construction of benzosultam motifs. As an example, the 6,7-dichlorobenzothiazine unit of a calpain inhibitor (Fig. 1a) requires six steps of synthesis before conjugation to 2-amino-3-phenyl-propanal.15 As direct construction of benzosultams by intermolecular cyclization is uncommon,16–18 synthesis of benzosultams often relies on intramolecular cyclization of elaborated precursors, whose preparation is often challenging.15,19–23 Despite recent advances, facile and efficient methods for the diversification and cyclization of peptidosulfonamides are still in demand.
Fig. 1.
Synthesis of peptidomimetics containing aryl sulfonamide motif. a Bioactive benzylsulfonamide and benzosultam-containing peptidomimetics. b Peptide-guided functionalization and macrocyclization of sulfonamide-containing peptidomimetics by Pd(II)-catalyzed late-stage C–H activation. HAT human airway trypsin-like protease
Transition metal-catalyzed C–H activation has shown great promise for the functionalization of amino acids and late-stage modification of peptides, as demonstrated by Yu,24–27 Lavilla/Albericio,28–30 Ackermann,31–33 and Daugulis,34 among others.35–41 Peptides have been shown to coordinate with palladium through amide bonds, promoting the functionalization of neighboring C–H bonds. Herein, we report a peptide-guided method for the functionalization of peptidosulfonamides by Pd(II)-catalyzed late-stage C–H activation. This reaction has broad substrate scope and provides facile access to a variety of bioactive benzylsulfonamide- and benzosultam-peptidomimetics, as well as peptidosulfonamide macrocycles (Fig. 1b). This strategyutilizes the N-sulfonated peptides in substrates as internal direacting groups and requires no external ligand or removable directing group. In addition, the reactions to generate benzosultam motifs follow a Pd(II)-catalyzed sequential C(sp2)–H activation mechanism, in which a second Civation mechanism, in whin unusual reactive peptide–Pd(II) complex. Furthermore, this reaction protocol allows efficient macrocyclization of substrates bearing acrylates or unactivated alkenes to produce bioactive peptidosulfonamide macrocycles.
Results
Olefination of benzylsulfonamide peptide conjugates
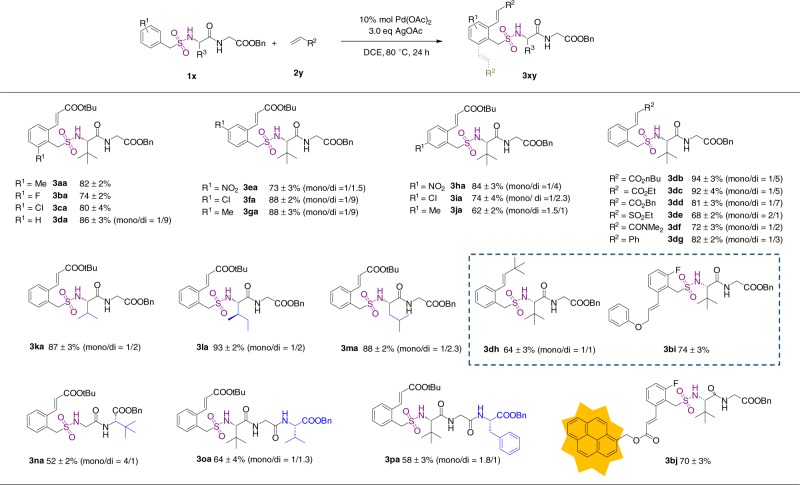
We initiated the investigation by evaluating the utility of dipeptide as a directing group to enable the olefination of benzylsulfonamides. To establish optimal reaction conditions, we employed an ortho-methylbenzylsulfonamide dipeptide conjugate 1a and tert-butyl acrylate 2a as substrates (Fig. 2). Detailed optimization studies reveal that the reaction proceeds most efficiently with 4.0 equiv of tert-butyl acrylate 2a in the presence of 10 mol% Pd(OAc)2 and 3.0 equiv of AgOAc in dichloroethane at 80 °C for 12 h, affording the ortho-olefination product 3aa in 80% isolated yield (Supplementary Table 1). The exocyclic double bond in product 3aa is determined to be E-configured by 1H-nuclear magnetic resonance (1H-NMR) analysis (Supplementary Figure 1). It is noteworthy that in contrast with previous reports of Pd-mediated olefination of arylsulfonamides,42,43 no external ligand is required in this procedure. Replacement of the N-sulfonated dipeptide by tLeu methyl ester (Supplementary Table 1, substrate 1a’) completely abolished the reaction under standard conditions, indicating that the dipeptide is required to enable the olefination reaction.
Fig. 2.
Scope of the olefination reaction of benzylsulfonamide peptide conjugates. The isolate yields were determined by three repeats of the experiments
Having established the optimal conditions, we sought to demonstrate the generality of this protocol with respect to the benzylsulfonyl group. As outlined in Fig. 2, substrates bearing ortho-halide-substitutions (1b, 1c) are fully tolerated in this protocol by yielding the corresponding olefination products 3ba and 3ca in 78% and 82% isolate yields, respectively. Reaction of substrate 1d gave dominantly the diolefination product 3da with an overall 88% yield. Substrates with para-substitutions, including nitro-, chloro-, and methyl groups, all underwent facile olefination and afford the corresponding products in excellent yields (3fa-3ga). Substrates with meta-substitutions (1h-1j) reacted with tert-butyl acrylate 2a smoothly; however, the yield of m-methylbenzylsulfonamide derivative 3j was noticeably lower (63%) and the mono-olefination product appeared as the major product.
Next, we examined the scope of reaction with respect to alkenes. Using benzylsulfonamide dipeptide conjugate 1d as the substrate, t-butyl, n-butyl, ethyl, and benzyl acrylate all react with substrate 1d in high yields, indicating that the steric property of acrylate substitutions does not affect the reaction efficiency (3db-3dd). The olefination proceeded in good yields when vinyl ethyl sulfone, N, N-dimethylacrylamide and styrene were employed (3de–3dg), demonstrating the versatility of this reaction. Catalytic C–H olefination reactions with unactivated, aliphatic alkenes are generally challenging due to their intrinsic poor reactivity.42–45 To our delight, unactivated alkenes 4-methylpent-1-ene and (allyloxy)benzene both reacted with high yields (3dh, 3bi), further expanding the substrate scope of this chemistry. The utility of this protocol is further highlighted by labeling a fluorinated peptidosulfonamide 3b with a fluorescent moiety in 70% isolated yield (3bj).
To extend the procedure to substrates with various peptide sequences, aliphatic amino acids, including Leu, Ile, and Val were placed at the N-terminus of the dipeptide. All substrates exhibited good reactivity and afforded diolefination products as the major products (Fig. 2, 3ka–3ma). Altering dipeptide sequence to Gly-tLeu in substrate 3n lowered the reaction efficiency and lead to dominantly mono-olefination in 53% yield (3na). Moreover, we prepared benzylsulfonamide-tripeptide conjugates 3o and 3p with tLeu-Gly-Val and tLeu-Gly-Phe to challenge the versatility of this procedure with regard to the length of the peptides. We were pleased to find that these tripeptides were also efficient in promoting the ortho-C–H olefination, and products 3oa and 3pa were isolated in 63% and 67% yield, respectively. Together, these results demonstrate that peptides are powerful directing groups for the preparation of benzylsulfonamide peptidomimetics.
To address the potential epimerization issue during the reaction, substrates 3q and 3q’ with dipeptides of d-tleu-Ala and L-tLeu-Ala were synthesized (Supplementary Fig. 2). We then conducted their reactions with acrylate 2a under standard conditions, and the stereochemistry of the resulting products was evaluated by NMR and high performance liquid chromatography (HPLC). Results showed that all substrates reacted efficiently with full conversion, affording corresponding products in high yields (Supplementary Fig. 2). Crude reaction mixtures of 3q and 3q’ (both mono- and di-substituted products) gave distinct retention times when analyzed by reversed phase-HPLC, indicating that the stereochemical integrity was retained and no epimerization occurred under the reaction conditions.
Cyclization of benzosulfonamide peptide conjugates
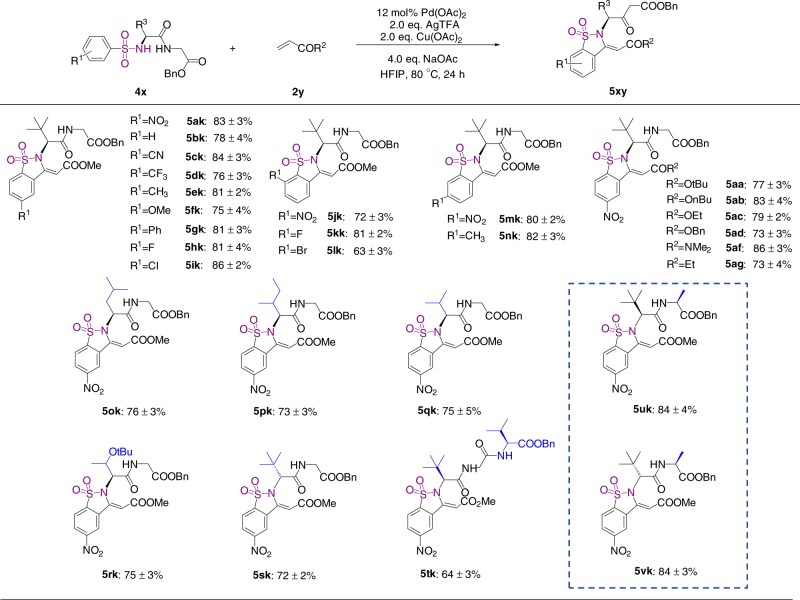
To further explore the generality of this peptide-guided strategy, we next examine the reaction of benzosulfonamide peptide conjugates by employing a p-nitrobenzene-sulfonamide dipeptide conjugate 4a and methyl acrylate 2k as substrates (Fig. 3). Interestingly, instead of ortho-olefination, we found that a benzosultam-dipeptide conjugate 5ak was generated as the major product, indicating that intermolecular cyclization has occurred. After extensively screening various parameters, we established that the treatment of substrate 4a with 4.0 equiv of methyl acrylate 2k, 12 mol% Pd(OAc)2 as the catalyst, along with 2.0 equiv of CF3COOAg, 2.0 equiv of Cu(OAc)2, and 4.0 equiv of NaOAc in hexafluoroisopropanol at 80 °C for 24 h, affording the cyclization product 5ak in 82% isolated yield (Supplementary Table 2). The requirement for a complex cocktail of metal reagents and base suggests that this reaction proceed through a distinct mechanism, which will be discussed in detail in the mechanism section.
Fig. 3.
Scope of the cyclization reaction of benzosulfonamide peptide conjugates. The isolate yields were determined by three repeats of experiments
With the optimized conditions in hand, we first examined the scope of this reaction with respect to the benzenesulfonyl group. As outlined in Fig. 3, substrates bearing electron-withdrawing groups such as nitrile and trifluoromethyl, or electron-donating groups such as methyl, methoxyl, and phenyl all react efficiently to afford the benzosultam-dipeptide conjugates (5ak–5gk) in high yields. Halide-substituted substrates are fully tolerated (5hk–5ik, 5kk–5lk), opening access to scaffolds that may be subject to further chemical manipulation. Ortho-, para-, and meta- substituents are all compatible with this procedure. Next, we evaluated the scope of acrylic acid esters (Fig. 3). Results show that ethyl acrylate, n-butyl acrylate, t-butyl, and benzyl acrylate all react with 4a with high yields (5aa–5ad), indicating that the steric properties of acrylate substitutions do not affect the reaction efficiency. The cyclization proceeded in good yields when N, N-dimethylacrylamide and ethyl vinyl ketone (5af–5ag) were employed, further demonstrating the versatility of this method. However, no benzosultam products were obtained when styrene and 4-methylpent-1-ene was employed as substrates, indicating that a conjugated carbonyl group is important for cyclization.
To extend the procedure to other peptide conjugates, Leu, Ile, Val, and t-Boc-protected Thr were placed at the N-terminus of the dipeptide. Gratifyingly, all these substrates showed good reactivity and underwent cyclization affording the desired products (Fig. 3, 5ok–5rk). For substrate 5s containing a D-tleu, the benzosultam formation was equally efficient as substrate 5a, indicating the chirality of amino acids in peptides has no impact on the reaction. Moreover, substrate 4t with tLeu-Gly-Val tripeptide reacted with methyl acrylate efficiently, affording product 5tk in 65% isolated yield. Together, these results demonstrate the versatility of this chemistry for the preparation of benzosultam peptidomimetics with peptides of various length and sequence.
To examine the potential epimerization during cyclization, substrates 4u and 4v with dipeptides of d-tleu-Ala and l-tLeu-Ala were synthesized and subject to reactions with methyl acrylate 2k under standard conditions (Fig. 3). HPLC analysis showed that both reactions proceeded highly efficiently with full conversion, yielding single epimeric products 5uk and 5vk (Supplementary Fig. 3). Thus, our protocol is compatible with peptides without epimerization and the chirality of dipeptides has no impact on its efficiency as a directing group.
Mechanism of benzosultam formation
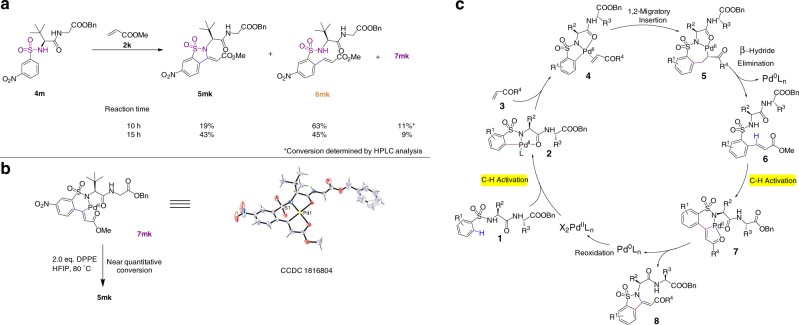
Previous reports of Pd- and Rh-catalyzed oxidative cyclization have generally proposed an aza-Wacker reaction following the initial olefination.46–48 To gain insight into the mechanism of benzosultam cyclization in our protocol, we monitored the reaction progress of substrates 4m and 2k at various time points by liquid chromatography–mass spectrometry (Fig. 4). After 10 h reaction, the starting material 4m was mostly consumed and converted to the corresponding olefination product 6mk in 63% yield, and the cyclization product 5mk in 19% yield. Prolonged reaction time resulted in accumulation of the cyclization product 5mk. Interestingly, an unexpected palladium-containing intermediate 7mk (~10% yield) was observed during the course of reaction. Both NMR and X-ray crystallographic analysis revealed that 7mk is a unique dipeptide–Pd(II) complex (Fig. 4b). In the structure of 7mk, the slightly distorted square planar Pd(II) atom is bonded to the palladated alkene carbon and carbonyl oxygen of the newly installed methyl acrylate, and the sulfonamide nitrogen and amide oxygen of the dipeptide, by forming a tricyclic structure. Together, these results suggest that a second C–H activation of the olefination product 6mk occurs and the dipeptide backbone directs the Pd(II) catalyst towards the proximal olefinic C(sp2)–H bond to generate the intermediate 7mk. Treatment of purified compound 7mk with 1, 2-bis(diphenylphosphino)-ethane (DPPE) (2.0 equiv) or reaction conditions omitting Pd catalyst resulted in near-quantitative conversion to the cyclization product 5mk, indicating that 7mk is a reactive intermediate for reductive elimination. In contrast, treatment of the olefination product 6mk under reaction conditions in absence of Pd catalysis leads to no reaction, showing that Pd is required for benzosultam cyclization. Consistent with these observations, reactions of substrates 4a, 4j, and 4o with methyl acrylate all generated the corresponding peptide–palladium complex 7ak, 7jk, and 7ok as intermediates (Supplementary Fig. 4). Further treatment of these Pd complexes with DPPE yields the corresponding cyclized benzosultam peptidomimetics, thereby establishing the general nature of a second C–H activation.
Fig. 4.
Mechanistic investigation of the benzosultam cyclization reaction. a Monitoring of the reaction progress at various time points by LC–MS. b X-ray crystallographic analysis of the Pd(II)–peptide complex 7mk and its conversion to product 5mk in the presence of DPPE. ORTEP representation of the crystal structure of 7mk. Gray = carbon, blue = nitrogen, purple = sulfur, yellow = Pd. Condition: 20 mol% Pd(OAc)2, 2.0 eq. AgTFA, 2.0 eq. Cu(OAc)2, 4.0 eq. AgOAc, HFIP, 80 °C. c Proposed catalytic cycle for olefination/oxidative cyclization
The overall catalytic cycle is therefore proposed to proceed as follows (Fig. 4c). Following the insertion of Pd(II) into the ortho-C–H bond directed by the N-sulfonated peptide, the resulting intermediate 2 coordinates with an olefin 3, and this is followed by 1,2-migratory insertion and β-hydride elimination, generating the olefination product 6. Subsequently, this uncyclized intermediate 6 associates with the Pd(II) catalyst again and undergoes a second C–H activation at the newly installed olefin by forming a tricyclic intermediate 7. Finally, reductive elimination of this Pd(II) complex intermediate 7 yields the benzosultam-dipeptide conjugate 8 as the final product. Reoxidation of Pd0Ln by Ag(I) or Cu(II) generates X2PdIILn catalyst to close the catalytic cycle. Our results provide an unusual example of cyclization of benzosulfonamide derivatives with olefins through a sequential C–H activation mechanism.
Self-guided macrocyclization of peptidosulfonamides
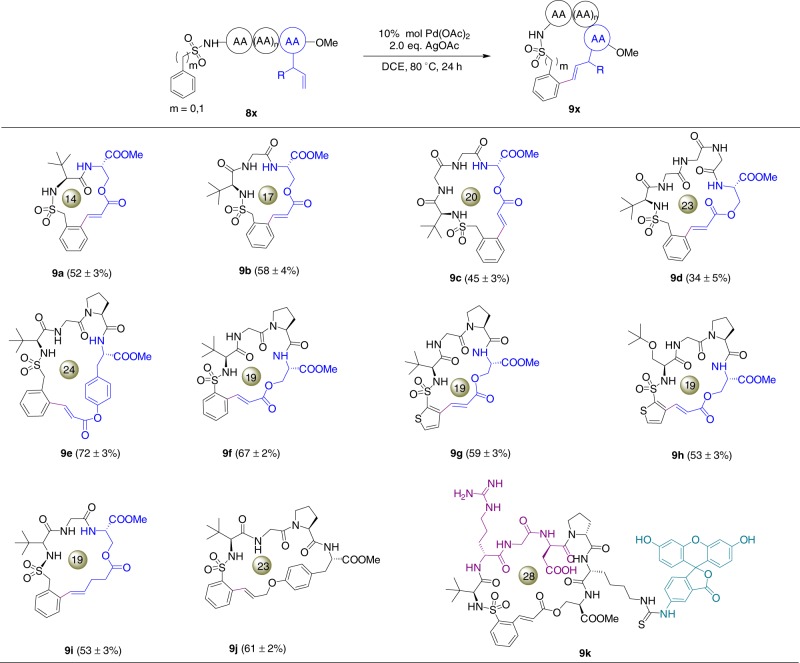
Cyclic peptides and peptide-based macrocycles have shown broad structural diversity and biological functions, however, chemical methods of constructing peptide macrocycles are currently limited.49-51 To further explore the applicability of our method, we conducted peptide macrocyclization to generate bioactive peptidosulfonamide macrocycles. We started with a benzylsulfonamide dipeptide conjugate 8a with the sequence of tLeu-O-acryloylserine-OMe (Fig. 5a). Gratifyingly, substrate 8a underwent efficient macrocyclization through o-olefination under standard conditions, affording the 14-membereddipeptide macrocycle 9a in 53% yield. Encouraged by this result, we continued to examine the macrocyclization of tripeptide, tetrapeptide and pentapeptide substrates 8b–8e. All substrates cyclized efficiently and provided 17- to 24-membered macrocycles 9b–9e in good yields, demonstrating the versatility of our strategy in constructing peptide macrocycles. Similarly, benzosulfonamide tetrapeptide conjugate 8f also cyclized under standard conditions, yielding a 20-membered macrocycle 9f.
Fig. 5.
Macrocyclization of peptidosulfonamides by self-guided C–H olefination. a Synthesis of peptidosulfonamide macrocycles. b FITC-labeled cyclic RGD peptide 9k and its integrin binding assays analyzed by confocal microscopy. All peptides were used at 2 µM concentration for the cell binding studies. The RGD motif is in red and the fluorescein motif is in green. The isolate yields were determined by three repeats of the experiments
We next challenged our method by introducing heterocycles into cyclic peptides. Thiophene is an important building block in a number of drugs and natural products.52 Thiophene-sulfonamide peptide conjugates 8g and 8h were then synthesized and subject to the standard procedure, and to our delight, macrocyclization was achieved in high yields through ortho-olefination of thiophene motifs, resulting in two 19-membered macrocycles 9g and 9h. The exocyclic double bond in product 9h is determined to be E-configured by 1H-NMR analysis (Supplementary Fig. 1). Olefination of benzylsulfonamides with unactivated alkenes is generally challenging and has not been reported in macrocyclization of peptides.53 Following our method, substrate 8i and 8j bearing unactivated alkene groups also underwent macrocyclization smoothly, further expanding the substrate scope of this protocol (Fig. 5). Together, these results indicate that the N-sulfonated peptide is powerful in self-guiding intramolecular C–H olefination. Following this self-guided peptide macrocyclization method, we proceeded to synthesize a fluorescent-labeled cyclic RGD peptide 9k with fluorescein isothiocyanate conjugated to a Lys residue (Supplementary Fig. 5). The RGD sequence is known for its selective binding to integrins when incorporated in appropriate cyclic structures.54 We examined the binding of compound 9k to U87MG cells, which is a glioblastoma cell line overexpressing the αvβ3 integrin. U87MG cells were then incubated with cyclic peptide 9k for 90 min before subjected to confocal microscopy analysis (Supplementary Fig. 6). Strong fluorescence staining was observed, indicating that the peptidosulfonamide macrocycle 9k exhibited binding affinity to the integrin. Together, these results demonstrate the applicability of our method in generating bioactive peptidosulfonamide macrocycles as molecular tools in biological systems.
Discussion
In summary, we have developed a peptide-guided method for functionalization and macrocyclization of bioactive peptidosulfonamides by Pd-catalyzed C–H activation. The potency of the peptides as a directing element is well demonstrated by the site-selective olefination of benzylsulfonamide with both activated and unactivated alkenes, as well as the cyclization of benzosulfonamide with acrylates. Moreover, our protocol allows macrocyclization of peptidosulfonamides bearing alkene functionalities. A RGD-containing peptidosulfonamide macrocycle exhibits strong binding affinity towards integrins, demonstrating the potential application of this chemistry in the development of pharmaceutical compounds and biological probes. The increasing significance of bioactive peptidomimetics containing pharmacophores should render this method attractive for synthetic and medicinal chemistry.
Methods
General information
All the reagents and solvents were obtained from Sigma-Aldrich, Alfa-Aesar or Acros, and used directly without further purification. Amino acids and derivatives were obtained from commercial sources. NMR spectra were recorded on Bruker AMX-400 instrument for 1H-NMR at 400 MHz and 13C NMR at 100 MHz, using TMS as internal standard. The following abbreviations (or combinations thereof) were used to explain multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad. Coupling constants J, are reported in Hertz units (Hz). High-resolution mass spectra (HRMS) were recorded on an Agilent Mass spectrometer using electrospray ionization-time of flight. HPLC profiles were obtained on Agilent 1260 HPLC system using commercially available columns.
General procedure for Pd-catalyzed reactions
Typically, the peptidosulfonamide substrates were placed in a 15 ml sealed reaction tube under indicated reaction conditions. The reaction mixture was stirred at 80 °C for 12–24 h, cooled to room temperature and then diluted with EtOAc (5.0 ml). The resulting solution was filtered through a Celite pad, concentrated under reduced pressure and the product was further purified by column chromatography and was typically obtained as a white solid.
Cell culture and staining experiments
U87MG cells were grown and maintained in DMEM media with 10% FBS and 1% penicillin/streptomycin at 37 °C, 5% CO2. Before staining experiments with peptides, the cells were seeded on the surface of MatTek glass bottom microwell dishes using 1 mL media. After 1 day, the cells were washed twice with warm DMEM media, incubated at 37 °C with 2 µM peptide 9k for 90 min and fixed. Images were taken using a Leica TCS SP8 confocal fluorescence microscope.
Data availability
X-ray crystallographic data of compound 7mk has been deposited in The Cambridge Crystallographic Data Centre (CCDC) with the Accession number of 1816804. All relevant data are available in supplementary information and from the authors.
Electronic supplementary material
Acknowledgments
This work is supported by the 1000-Youth Talents Plan, NSF of China (Grant 21778030), NSF of Jiangsu Province (Grant BK20160640) and the Fundamental Research Funds for the Central Universities (Grants 14380138 and 14380131). We thank Prof. Shaolin Zhu (NJU) and Prof. Zhuangzhi Shi (NJU) for helpful discussion during the preparation of the manuscript.
Author contributions
T.J. and H.C. designed and carried out most of the chemical reactions and analyzed the data. Y.H. and Q.B. supported the design and performance of synthetic experiments. W.S. performed the cell culture and staining experiments. H.W. designed and supervised the project. All authors discussed the results and commented on the manuscript. T.J., H.C., and H.W. wrote the manuscript. All authors have given approval to the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jian Tang, Hongfei Chen.
Change history
09/03/2018
This Article was originally published without the accompanying Peer Review File. This file is now available in the HTML version of the Article; the PDF was correct from the time of publication.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-05440-w.
References
- 1.Firer MA, Gellerman G. Targeted drug delivery for cancer therapy: the other side of antibodies. J. Hematol. Oncol. 2012;5:70. doi: 10.1186/1756-8722-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahrens VM, Bellmann-Sickert K, Beck-Sickinger AG. Peptides and peptide conjugates: therapeutics on the upward path. Future Med. Chem. 2012;4:1567–1586. doi: 10.4155/fmc.12.76. [DOI] [PubMed] [Google Scholar]
- 3.Drews J. Drug discovery: a historical perspective. Science. 2000;287:1960–1964. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]
- 4.Folkes A, et al. Design, synthesis and in vitro evaluation of potent, novel, small molecule inhibitors of plasminogen activator inhibitor-1. Bioorg. Med. Chem. Lett. 2002;12:1063–1066. doi: 10.1016/S0960-894X(02)00078-1. [DOI] [PubMed] [Google Scholar]
- 5.Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Anticancer and antiviral sulfonamides. Curr. Med. Chem. 2003;10:925–953. doi: 10.2174/0929867033457647. [DOI] [PubMed] [Google Scholar]
- 6.Zia-ur-Rehman M, Choudary JA, Ahmad S, Siddiqui HL. Synthesis of potential biologically active 1,2-benzothiazin-3-yl-quinazolin-4(3H)-ones. Chem. Pharm. Bull. 2006;54:1175–1178. doi: 10.1248/cpb.54.1175. [DOI] [PubMed] [Google Scholar]
- 7.Zhao XZ, et al. 2,3-dihydro-6,7-dihydroxy-1H-isoindol-1-one-based HIV-1 integrase inhibitors. J. Med. Chem. 2008;51:251–259. doi: 10.1021/jm070715d. [DOI] [PubMed] [Google Scholar]
- 8.Prakash GK, et al. Difluoro(sulfinato)methylation of N-sulfinyl imines facilitated by 2-pyridyl sulfone: stereoselective synthesis of difluorinated beta-amino sulfonic acids and peptidosulfonamides. Angew. Chem. Int. Ed. 2013;52:10835–10839. doi: 10.1002/anie.201304395. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer AJ, Liskamp RMJ. Synthesis of cyclic peptidosulfonamides by ring-closing metathesis. J. Org. Chem. 2004;69:3662–3668. doi: 10.1021/jo0358325. [DOI] [PubMed] [Google Scholar]
- 10.Wels B, Kruijtzer JAW, Garner KM, Adan RAH, Liskamp RMJ. Synthesis of cyclic peptidosulfonamides as scaffolds for MC4 pharmacophoric groups. Bioorg. Med. Chem. Lett. 2005;15:287–290. doi: 10.1016/j.bmcl.2004.10.082. [DOI] [PubMed] [Google Scholar]
- 11.Giordano C, et al. alpha-Peptide/beta-sulfonamidopeptide hybrids: analogs of the chemotactic agent for Met-Leu-Phe-OMe. Bioorg. Med. Chem. Lett. 2006;14:2642–2652. doi: 10.1016/j.bmc.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 12.Sielaff F, et al. Development of substrate analogue inhibitors for the human airway trypsin-like protease HAT. Bioorg. Med. Chem. Lett. 2011;21:4860–4864. doi: 10.1016/j.bmcl.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Kokotos G, et al. Inhibition of group IVA cytosolic phospholipase A2 by thiazolyl ketones in vitro, ex vivo, and in vivo. J. Med. Chem. 2014;57:7523–7535. doi: 10.1021/jm500192s. [DOI] [PubMed] [Google Scholar]
- 14.Azevedo CM, et al. Nonacidic free fatty acid receptor 4 agonists with antidiabetic activity. J. Med. Chem. 2016;59:8868–8878. doi: 10.1021/acs.jmedchem.6b00685. [DOI] [PubMed] [Google Scholar]
- 15.Wells GJ, Tao M, Josef KA, Bihovsky R. 1,2-Benzothiazine 1,1-dioxide P(2)-P(3) peptide mimetic aldehyde calpain I inhibitors. J. Med. Chem. 2001;44:3488–3503. doi: 10.1021/jm010178b. [DOI] [PubMed] [Google Scholar]
- 16.Miura T, Yamauchi M, Kosaka A, Murakami M. Nickel-catalyzed regio- and enantioselective annulation reactions of 1,2,3,4-benzothiatriazine-1,1(2H)-dioxides with allenes. Angew. Chem. Int. Ed. 2010;49:4955–4957. doi: 10.1002/anie.201001918. [DOI] [PubMed] [Google Scholar]
- 17.Thrimurtulu N, Nallagonda R, Volla CMR. Cobalt-catalyzed aryl C–H activation and highly regioselective intermolecular annulation of sulfonamides with allenes. Chem. Comm. 2017;53:1872–1875. doi: 10.1039/C6CC08622E. [DOI] [PubMed] [Google Scholar]
- 18.Pham MV, Ye B, Cramer N. Access to sultams by rhodium(III)-catalyzed directed C–H activation. Angew. Chem. Int. Ed. 2012;51:10610–10614. doi: 10.1002/anie.201206191. [DOI] [PubMed] [Google Scholar]
- 19.Ruppel JV, Kamble RM, Zhang XP. Cobalt-catalyzed intramolecular C–H amination with arylsulfonyl azides. Org. Lett. 2007;9:4889–4892. doi: 10.1021/ol702265h. [DOI] [PubMed] [Google Scholar]
- 20.Zeng W, Chemler SR. Copper(II)-catalyzed enantioselective intramolecular carboamination of alkenes. J. Am. Chem. Soc. 2007;129:12948–12949. doi: 10.1021/ja0762240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rousseaux S, Gorelsky SI, Chung BK, Fagnou K. Investigation of the mechanism of C(sp3)–H bond cleavage in Pd(0)-catalyzed intramolecular alkane arylation adjacent to amides and sulfonamides. J. Am. Chem. Soc. 2010;132:10692–10705. doi: 10.1021/ja103081n. [DOI] [PubMed] [Google Scholar]
- 22.Ichinose M, et al. Enantioselective intramolecular benzylic C–H bond amination: efficient synthesis of optically active benzosultams. Angew. Chem. Int. Ed. 2011;50:9884–9887. doi: 10.1002/anie.201101801. [DOI] [PubMed] [Google Scholar]
- 23.Ishida N, Shimamoto Y, Yano T, Murakami M. 1,5-Rhodium shift in rearrangement of N-arenesulfonylazetidin-3-ols into benzosultams. J. Am. Chem. Soc. 2013;135:19103–19106. doi: 10.1021/ja410910s. [DOI] [PubMed] [Google Scholar]
- 24.Gong W, Zhang G, Liu T, Giri R, Yu JQ. Site-selective C(sp3)–H functionalization of di-, tri-, and tetrapeptides at the N-terminus. J. Am. Chem. Soc. 2014;136:16940–16946. doi: 10.1021/ja510233h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Qiao JX, Poss MA, Yu JQ. Palladium(II)-catalyzed site-selective C(sp3)–H alkynylation of oligopeptides: a linchpin approach for oligopeptide-drug conjugation. Angew. Chem. Int. Ed. 2017;56:10924–10927. doi: 10.1002/anie.201706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G, et al. Ligand-enabled beta-C–H arylation of alpha-amino acids without installing exogenous directing groups. Angew. Chem. Int. Ed. 2017;56:1506–1509. doi: 10.1002/anie.201610580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He J, et al. Ligand-controlled C(sp(3))–H arylation and olefination in synthesis of unnatural chiral alpha-amino acids. Science. 2014;343:1216–1220. doi: 10.1126/science.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noisier AF, Garcia J, Ionut IA, Albericio F. Stapled peptides by late-stage C(sp3)-H activation. Angew. Chem. Int. Ed. 2017;56:314–318. doi: 10.1002/anie.201608648. [DOI] [PubMed] [Google Scholar]
- 29.Mendive-Tapia L, et al. New peptide architectures through C–H activation stapling between tryptophan-phenylalanine/tyrosine residues. Nat. Comm. 2015;6:7160. doi: 10.1038/ncomms8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendive-Tapia L, et al. Constrained cyclopeptides: biaryl formation through Pd-catalyzed C–H activation in peptides-structural control of the cyclization vs. cyclodimerization outcome. Chemistry. 2016;22:13114–13119. doi: 10.1002/chem.201601832. [DOI] [PubMed] [Google Scholar]
- 31.Schischko A, Ren H, Kaplaneris N, Ackermann L. Bioorthogonal diversification of peptides through selective ruthenium(II)-catalyzed C–H activation. Angew. Chem. Int. Ed. 2017;56:1576–1580. doi: 10.1002/anie.201609631. [DOI] [PubMed] [Google Scholar]
- 32.Ruan Z, Sauermann N, Manoni E, Ackermann L. Manganese-catalyzed C–H alkynylation: expedient peptide synthesis and modification. Angew. Chem. Int. Ed. 2017;56:3172–3176. doi: 10.1002/anie.201611118. [DOI] [PubMed] [Google Scholar]
- 33.Bauer M, Wang W, Lorion ML, Dong C, Ackermann L. Internal peptide late-stage diversification: peptide isosteric triazoles for primary and secondary C(sp3)–H activation. Angew. Chem. Int. Ed. 2017;56:1–6. doi: 10.1002/anie.201610955. [DOI] [PubMed] [Google Scholar]
- 34.Tran LD, Daugulis O. Nonnatural amino-acid synthesis by using carbon-hydrogen bond functionalization methodology. Angew. Chem. Int. Ed. 2012;51:5188–5191. doi: 10.1002/anie.201200731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang J, He YD, Chen HF, Sheng WJ, Wang H. Synthesis of bioactive and stabilized cyclic peptides by macrocyclization using C(sp(3))–H activation. Chem. Sci. 2017;8:4565–4570. doi: 10.1039/C6SC05530C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B, et al. Palladium-catalyzed stereoretentive olefination of unactivated C(sp3)-H bonds with vinyl iodides at room temperature: synthesis of beta-vinyl alpha-amino acids. Org. Lett. 2014;16:6260–6263. doi: 10.1021/ol503248f. [DOI] [PubMed] [Google Scholar]
- 37.Liu YJ, et al. Divergent and stereoselective synthesis of beta-silyl-alpha-amino acids through palladium-catalyzed intermolecular silylation of unactivated primary and secondary C–H bonds. Angew. Chem. Int. Ed. 2016;55:13859–13862. doi: 10.1002/anie.201607766. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Yin XS, Chen K, Zhang SQ, Shi BF. Stereoselective synthesis of chiral beta-fluoro alpha-amino acids via Pd(II)-catalyzed fluorination of unactivated methylene C(sp(3))–H bonds: scope and mechanistic studies. J. Am. Chem. Soc. 2015;137:8219–8226. doi: 10.1021/jacs.5b03989. [DOI] [PubMed] [Google Scholar]
- 39.Wencel-Delord J, Glorius F. C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 2013;5:369–375. doi: 10.1038/nchem.1607. [DOI] [PubMed] [Google Scholar]
- 40.Chalker JM, Bernardes GJL, Davis BG. A “Tag-and-Modify” approach to site-selective protein modification. Acc. Chem. Res. 2011;44:730–741. doi: 10.1021/ar200056q. [DOI] [PubMed] [Google Scholar]
- 41.Noisier AF, Brimble MA. C–H functionalization in the synthesis of amino acids and peptides. Chem. Rev. 2014;114:8775–8806. doi: 10.1021/cr500200x. [DOI] [PubMed] [Google Scholar]
- 42.Manoharan R, Sivakumar G, Jeganmohan M. Cobalt-catalyzed C–H olefination of aromatics with unactivated alkenes. Chem. Comm. 2016;52:10533–10536. doi: 10.1039/C6CC04835H. [DOI] [PubMed] [Google Scholar]
- 43.Yi CS, Lee DW. Regioselective intermolecular coupling reaction of arylketones and alkenes involving C–H bond activation catalyzed by an in-situ formed cationic ruthenium-hydride complex. Organometallics. 2009;28:4266–4268. doi: 10.1021/om900416k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sevov CS, Hartwig JF. Iridium-catalyzed oxidative olefination of furans with unactivated alkenes. J. Am. Chem. Soc. 2014;136:10625–10631. doi: 10.1021/ja504414c. [DOI] [PubMed] [Google Scholar]
- 45.Lu MZ, Chen XR, Xu H, Dai HX, Yu JQ. Ligand-enabled ortho-C–H olefination of phenylacetic amides with unactivated alkenes. Chem. Sci. 2018;9:1311–1316. doi: 10.1039/C7SC04827K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu Y, Wang DH, Engle KM, Yu JQ. Pd(II)-catalyzed hydroxyl-directed C–H olefination enabled by monoprotected amino-acid ligands. J. Am. Chem. Soc. 2010;132:5916–5921. doi: 10.1021/ja101909t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang H, He J, Liu T, Yu JQ. Ligand-enabled gamma-C(sp(3))–H olefination of amines: en route to pyrrolidines. J. Am. Chem. Soc. 2016;138:2055–2059. doi: 10.1021/jacs.5b13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi Z, Schroder N, Glorius F. Rhodium(III)-catalyzed dehydrogenative Heck reaction of salicylaldehydes. Angew. Chem. Int. Ed. 2012;51:8092–8096. doi: 10.1002/anie.201203224. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, et al. A general strategy for synthesis of cyclophane-braced peptide macrocycles via palladium-catalysed intramolecular sp(3) C–H arylation. Nat. Chem. 2018;10:540–548. doi: 10.1038/s41557-018-0006-y. [DOI] [PubMed] [Google Scholar]
- 50.Zorzi A, Deyle K, Heinis C. Cyclic peptide therapeutics: past, present and future. Curr. Opin. Chem. Biol. 2017;38:24–29. doi: 10.1016/j.cbpa.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 51.White CJ, Yudin AK. Contemporary strategies for peptide macrocyclization. Nat. Chem. 2011;3:509–524. doi: 10.1038/nchem.1062. [DOI] [PubMed] [Google Scholar]
- 52.Gramec D, Peterlin Masic L, Sollner Dolenc M. Bioactivation potential of thiophene-containing drugs. Chem. Res Toxicol. 2014;27:1344–1358. doi: 10.1021/tx500134g. [DOI] [PubMed] [Google Scholar]
- 53.Lu MZ, Chen XR, Xu H, Dai HX, Yu JQ. Ligand-enabled ortho-C−H olefination of phenylacetic amides with unactivated alkenes. Chem. Sci. 2018;9:1311–1316. doi: 10.1039/C7SC04827K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med. Chem. 2010;10:753–768. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
X-ray crystallographic data of compound 7mk has been deposited in The Cambridge Crystallographic Data Centre (CCDC) with the Accession number of 1816804. All relevant data are available in supplementary information and from the authors.