Abstract
Background
The purpose of this study was to evaluate the functional outcomes, infection rate, and complications associated with shoulder arthroplasty for sequelae of prior septic arthritis.
Methods
This is a retrospective cohort study of 17 patients who underwent shoulder arthroplasty for sequelae of septic arthritis. Patients were analyzed for patient-reported outcomes, complications, and reoperations.
Results
The 17 patients in this cohort were an average age of 65.4 ± 12.2 years old, were 58.8% male, and had an average body mass index of 27.9 ± 4.1 kg/m2. These patients underwent 14 reverse shoulder arthroplasties (RSAs; 11 after antibiotic spacer placement), one anatomic total shoulder arthroplasty after antibiotic spacer placement, and two hemiarthroplasties (both after antibiotic spacer placement). Two patients underwent reoperation (dislocated RSAs). There were four complications (23.5%): two RSA dislocations, one acromial stress fracture, and one atraumatic rotator cuff tear after hemiarthroplasty. There were no cases of postoperative wound complications or infection. At an average of 4.1 ± 1.8 years of follow-up for all 17 of 17 cases, the average visual analogue scale pain score was 4.6 ± 2.3, average Single Assessment Numeric Evaluation Score was 59.3 ± 23.7, average American Shoulder and Elbow Surgeons Score was 57.6 ± 15.5, and average Simple Shoulder Test was 6.9 ± 2.6 based on “yes” responses.
Conclusions
Shoulder arthroplasty after septic arthritis had inconsistent functional outcomes and high complication rates but no reinfection.
Keywords: Shoulder replacement arthroplasty, Infectious arthritis, Complications
Native septic arthritis of the glenohumeral joint is an uncommon but potentially devastating condition.1,2,3) Particularly, end-stage postinfectious glenohumeral arthritis can lead to significant pain and disability.4) While there are a number of studies focusing on arthroplasty options for the sequelae of septic arthritis in the lower extremity5,6,7,8,9,10,11) and glenohumeral periprosthetic joint infection,12,13,14,15,16,17,18) results of shoulder arthroplasty for postinfectious glenohumeral arthrosis have been less commonly reported.
There have been a small number of case series on the results of shoulder arthroplasty for the sequelae of prior septic arthritis.4,19,20) While these studies found low reinfection rates, patients sustained high complication and reoperation rates with marginally improved functional scores. Patients in these studies were mostly treated prior to the increased utilization of reverse shoulder arthroplasty (RSA),21,22,23) with only one study of eight patients utilizing an RSA implant for native, end-stage postinfectious glenohumeral arthritis.19) Given the clinical difficulty of treating this population and the limited treatment options available, we aimed to describe our institutional experience with shoulder arthroplasty in this population. We hypothesized that these patients uncommonly experience recurrence of infection and will still have mediocre clinical outcomes despite utilization of RSA.
METHODS
Following approval by the Institutional Review Board of Thomas Jefferson University Hospital, all shoulder arthroplasties were identified by querying of an institutional shoulder arthroplasty database by International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM). The time period over which this data was collected was January 2010 through September 2015. The ICD-9-CM codes utilized were 81.80 (anatomic total shoulder arthroplasty [aTSA]), 81.81 (partial shoulder arthroplasty [hemiarthroplasty]), and 81.88 (RSA). Direct chart review was performed on all patients to obtain the diagnosis at the time of shoulder arthroplasty. All patients who underwent arthroplasty for the sequelae of septic arthritis (pain and shoulder dysfunction in the setting of prior infectious arthritis) were included. Arthroplasty was performed after eradication of the infection either through debridement or placement of an antibiotic spacer and a course of antibiotics. These patients were not undergoing arthroplasty as a treatment for a current or acute infection, they were undergoing arthroplasty for the sequelae of septic arthritis in which the infection had already been eradicated. Search was performed of our institutional arthroplasty database rather than individuals that were coded for a diagnosis of primary septic arthritis for two reasons. Firstly, in our institutional database, procedures are coded more reliably than diagnoses. Secondly, all arthroplasty cases were directly chart reviewed, so all arthroplasty cases performed after septic arthritis were known to be captured. Patients that underwent revision arthroplasty for periprosthetic joint infection were not included. Those that underwent arthroplasty after an index antibiotic spacer placement for septic arthritis or osteomyelitis of the native shoulder were included in this cohort.
Demographic factors such as age, sex, body mass index (BMI), and age adjusted Charlson Comorbidity Index were recorded.24) Direct chart review was performed on these patients to identify postoperative complications, reinfection, reoperation, and range of motion (ROM; at a minimum of 1 year after surgery). Additionally, patients were contacted at a minimum of 2 years after surgery for patient-reported outcomes. The outcomes analyzed were visual analogue scale (VAS) pain score, Single Assessment Numeric Evaluation (SANE) score,25) American Shoulder and Elbow Surgeons (ASES) score,26) and Simple Shoulder Test (SST) score.27)
Per protocol at our institution, after a standardized skin preparation and surgical exposure, the glenohumeral joint was aspirated with a needle prior to arthrotomy. This synovial fluid was sent for culture. Once the joint was opened, tissue from the anterior capsule, inferior capsule, glenoid, and humeral canal, were sent for culture. Each culture was placed directly into sterile specimen containers with previously unused instruments. Specimens were held for 2 weeks and sent for both aerobic and anaerobic cultures. All procedures were performed at one hospital with one microbiology laboratory. All patients received a standard regimen of 2 weeks of antibiotics postoperatively until the cultures were finalized as negative. The specific antibiotic utilized was based on the susceptibilities of the organism isolated from previous surgeries. If patients had positive cultures, they were kept on antibiotics for at least 6 weeks with routine clinical follow-up for signs and symptoms of infection by both the surgical team and the infectious diseases service at our institution. The decision to undergo arthroplasty after placement of an antibiotic spacer was based on clinical improvement and normalization of inflammatory laboratory results (erythrocyte sedimentation rate and C-reactive protein), and culture status from most recent surgery or aspiration. All cases were performed by one of five shoulders and elbow fellowship trained surgeons. Fifteen of 17 cases were done by a surgeon that has been in practice for over 20 years. One case was done by a surgeon in practice for over 10 years and the final case was done by a surgeon 5 years into practice. The specific implant utilized was selected by surgeon's preference. These systems were as follows: Zimmer Biomet (Warsaw, IN, USA), DePuy Synthes (Warsaw, IN, USA), DJO Global (Vista, CA, USA), and Wright Medical (Memphis, TN, USA). Clinical follow-up after final arthroplasty was performed at 2, 6, 12 weeks, 6 months, and 1 year with the treating surgeon and up to a year of follow-up with the infectious disease team (depending on the treatment protocol) for monitoring of recurrent infection.
Descriptive statistics (mean, standard deviation, minimum, and maximum) were calculated for demographic factors, postoperative complications, and patientreported outcomes. Comparison of proportions by z scores was conducted for categorical variables while two sample t-test assuming unequal variance was conducted for continuous variables. All statistics were calculated with Microsoft Excel 2013 (Microsoft, Redmond, WA, USA).
RESULTS
There were 17 patients identified over the 5-year study period that underwent shoulder arthroplasty for the sequelae of septic arthritis. The average age was 65.4 ± 12.2 years (range, 46.2 to 81.3 years). This population was 58.8% male. The average BMI was 27.9 ± 4.1 kg/m2 (21.1 to 36.9 kg/m2). The average age adjusted Charlson Comorbidity Index was 3.5 ± 1.5 (range, 1 to 6). Comparatively, over this same time period, there were 2,463 primary shoulder arthroplasties and 360 revision shoulder arthroplasties performed at the same institution. The 2,463 primary patients had a similar preoperative demographic composition to the study population. They had an average age of 67.4 ± 11.1 years (p = 0.99), BMI of 30.3 ± 6.7 kg/m2 (p = 0.97), age-adjusted Charlson Comorbidity Index of 3.7 ± 1.5 (p = 0.99) and were 47.2% male (p = 0.34). The most frequent medical comorbidities in the study population were chronic pulmonary disease (4/17, 23.5%), rheumatic disease (2/17, 11.8%), cardiovascular disease (1/17, 5.9%), chronic kidney disease (1/17, 5.9%), and diabetes with complications (1/17, 5.9%). Twelve of the 17 patients (70.6%) had previous surgery before their infection (Table 1). Overall, 16 patients had rotator cuff dysfunction at the time of arthroplasty (14 irreparable tears, one repairable tear, and one greater tuberosity malunion).
Table 1. Previous Surgical History for the Population That Underwent Shoulder Arthroplasty for Infection.
| Patient | Sex | Age (yr) | Surgery before infection | Surgery for infection | Culture | Clinical detail |
|---|---|---|---|---|---|---|
| 1 | Male | 61.93 | 2 | 4 | Staphylococcus epidermidis | Two failed open cuff repairs with infection treated with Keflex before presentation; four I&Ds |
| 2 | Female | 74.91 | 0 | 2 | MSSA | Multifocal septic native arthritis, one shoulder I&D |
| 3 | Male | 52.68 | 1 | 3 | Staphylococcus epidermidis | One open rotator cuff repair, developed sinus tract 5 years later, two I&Ds before presentation |
| 4 | Female | 64.78 | 5 | 1 | CNS, Propionibacterium acnes | Five attempted cuff repairs with symptom recurrence before presentation (three scope, two open); one I&D |
| 5 | Male | 76.85 | 3 | 1 | Unknown (OSH) | Rotator cuff surgery three times before presentation; one I&D |
| 6 | Male | 52.06 | 1 | 2 | CNS, Propionibacterium acnes | Failed open cuff repair before presentation; two I&Ds |
| 7 | Female | 70.83 | 5 | 2 | CNS | Distal clavicle excision with frozen shoulder, four MUA and pain pump placed, infected and washed out before presentation |
| 8 | Female | 79.88 | 1 | 2 | Staphylococcus lugdunensis, Escherichia coli | One scope cuff repair, infected and open I&D before presentation |
| 9 | Male | 57.78 | 0 | 4 | MRSA | Two injections, septic arthritis with septic emboli to brain and endocarditis, three I&Ds before presentation |
| 10 | Female | 69.25 | 1 | 4 | CNS, Propionibacterium acnes | One cuff repair and capsular release, two I&Ds for infection before presentation; one antibiotic spacer before 2-stage |
| 11 | Male | 47.75 | 2 | 1 | Staphylococcus epidermidis | Two scope labral repairs before presentation; rapidly progressive glenohumeral arthrosis |
| 12 | Male | 80.98 | 0 | 4 | MRSA, Pseudomonas aeruginosa | Nonoperative proximal humerus malunion, three injections; went for delayed RSA, but I&D instead |
| 13 | Female | 77.19 | 0 | 2 | MSSA | One injection for cuff tear arthropathy and plan for RSA, clinically infected intraoperatively, spacer placed |
| 14 | Female | 65.13 | 2 | 1 | CNS | Failed proximal humerus plate and revised to intramedullary nail, failed before presentation |
| 15 | Male | 81.29 | 0 | 1 | Escherichia coli | One injection, worsened and had scope I&D before presentation |
| 16 | Male | 52.52 | 2 | 2 | Unknown (OSH) | Two failed rotator cuff repairs, purulent drainage with I&D before presentation |
| 17 | Male | 46.19 | 1 | 2 | Enterobacter agglomerans | Failed proximal humerus plate with collapse and septic arthritis |
I&D: irrigation and debridement, MSSA: methicillin-sensitive Staphylococcus aureus, CNS: coagulase-negative Staphylococcus aureus, OSH: outside hospital, MUA: manipulation under anesthesia, MRSA: methicillin-resistant Staphylococcus aureus, RSA: reverse shoulder arthroplasty.
These 17 patients were treated definitively with 14 RSA (82.4%; 11 after an index resection and placement of an antibiotic spacer, three after a single irrigation and debridement), one aTSA (5.9%; after a resection and placement of an antibiotic spacer), and two hemiarthroplasties (11.8%; both after a resection and placement of an antibiotic spacer). The 14 patients that underwent RSA were treated with a reverse implant due to an irreparable and atrophied rotator cuff. The one patient that underwent aTSA was treated with an anatomic implant in the setting of an intact rotator cuff. The two patients treated with a hemiarthroplasty had supraspinatus tears that were repaired intraoperatively. Utilization of an antibiotic spacer was determined by concern for underlying osteomyelitis or in the setting of a planned arthroplasty following initial treatment for septic arthritis. The presence of osteomyelitis was identified on preoperative imaging or by clinical concern intraoperatively. Timing of reimplantation was based on clinical improvement and normalization of inflammatory laboratory results (erythrocyte sedimentation rate and C reactive protein). Thirteen of the 17 patients underwent arthroplasty through a deltopectoral approach (76.5%) while four of 17 (33.5%) underwent a superior approach because of prior surgical incisions. There was substantial heterogeneity with regards to rotator cuff integrity, stem length, and utilization of cement (Table 2, Figs. 1 and 2).
Table 2. Surgical Details for Each Patient.
| Patient | Procedure performed | Rotator cuff status | Two-stage procedure | Time between two stages (wk) | Surgical approach | Long versus standard length stem | Antibiotic cement | Culture from arthroplasty surgery |
|---|---|---|---|---|---|---|---|---|
| 1 | RSA | Irreparable, atrophied cuff | No | NA | Superior | Standard | Yes | Negative |
| 2 | RSA | Irreparable, atrophied cuff | Yes | 6 | Deltopectoral | Long | Yes | Negative |
| 3 | Hemiarthroplasty | Torn supraspinatus, repaired | Yes | 19 | Deltopectoral | Standard | Uncemented | Staphylococcus epidermidis, Propionibacterium acnes |
| 4 | RSA | Irreparable, atrophied cuff | No | NA | Superior | Standard | Yes | Negative |
| 5 | RSA | Irreparable, atrophied cuff | No | NA | Superior | Standard | Yes | Negative |
| 6 | RSA | Irreparable, atrophied cuff | No | NA | Superior | Standard | Yes | Micrococcus |
| 7 | RSA | Irreparable, atrophied cuff | Yes | 3 | Deltopectoral | Standard | Yes | Negative |
| 8 | RSA | Irreparable, atrophied cuff | Yes | 14 | Deltopectoral | Standard | Uncemented | Negative |
| 9 | RSA | Irreparable, atrophied cuff | Yes | 4 | Deltopectoral | Standard | Uncemented | Negative |
| 10 | RSA | Irreparable, atrophied cuff | Yes | 36 | Deltopectoral | Standard | Yes | Coagulase-negative Staphylococcus |
| 11 | aTSA | Intact | Yes | 5 | Deltopectoral | Standard | Uncemented | Staphylococcus epidermidis, Propionibacterium acnes |
| 12 | RSA | Irreparable, atrophied cuff | Yes | 4 | Deltopectoral | Standard | Uncemented | Negative |
| 13 | RSA | Irreparable, atrophied cuff | Yes | 18 | Deltopectoral | Standard | Yes | Negative |
| 14 | RSA | Tuberosity malunion | Yes | 6 | Deltopectoral | Standard | Uncemented | Negative |
| 15 | RSA | Irreparable, atrophied cuff | Yes | 15 | Deltopectoral | Standard | Yes | Negative |
| 16 | Hemiarthroplasty | Torn supraspinatus, repaired (poor quality) | Yes | 52 | Deltopectoral | Standard | Uncemented | Negative |
| 17 | RSA | Irreparable, atrophied cuff | Yes | 15 | Deltopectoral | Standard | Yes | Negative |
RSA: reverse shoulder arthroplasty, NA: not applicable, aTSA: anatomic total shoulder arthroplasty.
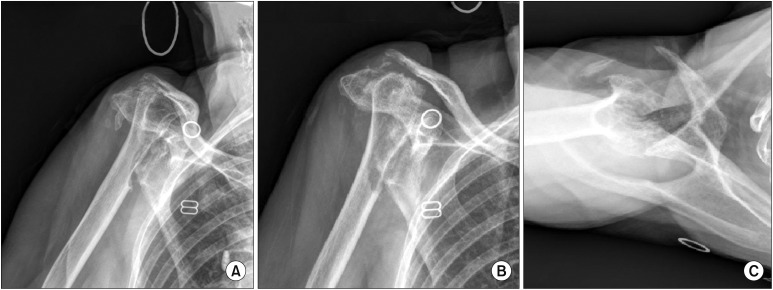
Fig. 1. Preoperative true anteroposterior (AP) (A), AP (B), and axillary (C) radiographs of an 81-year-old female who had multifocal septic native arthritis with one previous shoulder irrigation and debridement.
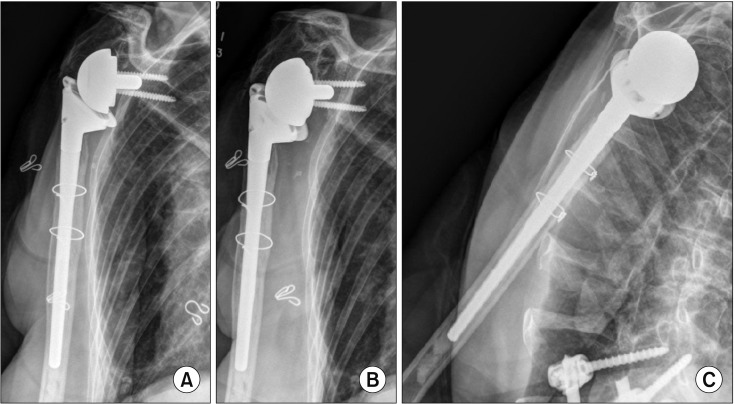
Fig. 2. Three-year postoperative true anteroposterior (AP) (A), AP (B), and scapular Y (C) views of an 81-year-old female who had multifocal septic native arthritis with one previous shoulder irrigation and debridement and underwent a two-staged reverse shoulder arthroplasty.
Regarding outcomes, two of 17 patients (11.7%) underwent reoperation at 28 and 64 days postoperatively. The first patient sustained an atraumatic RSA dislocation and underwent revision of the glenoid and humeral components that were grossly loose intraoperatively. Intraoperative cultures for this case were negative. The second patient also sustained an atraumatic RSA dislocation and underwent revision to a thicker polyethylene component. There were four complications (23.5%): two aforementioned dislocations, one acromial stress fracture, and one atraumatic rotator cuff tear after hemiarthroplasty (patient elected not to undergo conversion to RSA). There were no cases of postoperative wound complications or reinfection. Ten of the 14 patients that underwent RSA (71.4%) had ROM data available at a minimum 1-year follow-up (mean, 2.2 ± 1.7 years). In this cohort, the forward elevation was 110° ± 30.2° (60° to 150°). Regarding patient-reported outcomes, all 17 patients achieved over a 2-year follow-up. The average follow-up was 4.1 ± 1.8 years (range, 2 to 8.3 years) for all 17 cases. Average VAS pain score was 4.6 ± 2.3 (range, 0 to 8), average SANE score was 59.3 ± 23.7 (range, 0 to 95), average ASES score was 57.6 ± 15.5 (range, 26.7 to 83.3), and average SST score was 6.9 ± 2.6 (range, 1 to 10) “yes” responses.
DISCUSSION
This analysis reviewed the results of shoulder arthroplasty in 17 patients with end-stage, postinfectious glenohumeral arthritis at mid-term follow-up. RSA was the most common treatment choice. There were no recurrent infections and two patients required further surgery. However, nearly one quarter of the population sustained a complication (two RSA dislocations, one acromial stress fracture, and one rotator cuff tear after hemiarthroplasty) and functional results were mediocre. These complications were likely a result of the poor quality of the soft tissue envelope and highlight the difficulty of balancing soft tissue tension in the setting of multiple previous surgeries as well as the local destruction of the antecedent infection and initial debridement. The index infection was treated aggressively in this population. Patients with end-stage post-septic arthritis and concern for underlying osteomyelitis were treated with multiple debridements and resection arthroplasty with placement of an antibiotic spacer. All patients were followed by infectious disease specialists perioperatively and did not undergo arthroplasty until clinical and serological resolution of concern for infection. This aggressive approach may have contributed to the reinfection rate of zero at our institution.
There was only one previous study that included RSA in treatment of end-stage postinfectious glenohumeral arthritis that was performed by Morris et al.19) and evaluated results in eight patients. While seven of eight patients were satisfied with their clinical results and there were no recurrent infections at an average of 4.4 years of follow-up, the complication rate was 37.5%. Mean postoperative ASES and SANE scores in the analysis by Morris et al.19) were 78.4 and 59.9, respectively. We reported a similar SANE score of 59.3 and a complication rate of 23.5% to Morris et al.19) Schoch et al.20) performed the largest case series that analyzed a population of 23 shoulders treated with either aTSA or hemiarthroplasty for end-stage postinfectious glenohumeral arthritis. This study reported a 34.8% complication rate, 21.7% reoperation rate, and a reinfection rate of 8.7%. Active forward elevation was 112° in this series of anatomic arthroplasties which is similar to the 110° that we report with use of RSA. A direct comparison between studies is difficult to interpret because in the study by Schoch et al.,20) seven of the 23 shoulders (30.4%) were found to have rotator cuff tears, while the vast majority (94%) of the patients in our study with sequelae of prior septic arthritis had rotator cuff dysfunction. Our analysis and these prior studies show consistently limited functional results, but a reasonably low reinfection rate with only two reinfections across all studies. In knee arthroplasty, similar potential for infection eradication has been found with two-stage treatment.9) These patients showed more improved functional outcomes relative to shoulder infection patients. As antibiotic spacer techniques evolve further, functional outcomes of second-stage arthroplasty in the shoulder may also improve.28)
The results of this study must be considered in the context of its limitations. This is a purely retrospective study and therefore is subject to all limitations of a retrospective study. The ROM data was particularly limited as forward elevation was the only consistently measured data point and this was only available in 10 of 17 of this population at 1 year. Additionally, patient-reported outcomes were not available preoperatively and so could not be compared to postoperative outcomes scores. Therefore, it is difficult to state how much patients improved with their final functional scores. Finally, the treatment cohort is admittedly heterogeneous with substantial variability in terms of prior surgical procedures, rotator cuff integrity, bone loss, and other factors that could influence outcome. Finally, these patients only represent a small proportion of our entire arthroplasty population. Therefore, any meaningful analysis of outcomes between these patients and other arthroplasty patients would be limited by different population size and composition. Despite this, we believe that the heterogeneity apparent in this study reflects the true clinical experience with this challenging patient population.
Postinfectious arthritis of the shoulder is an uncommon problem and treatment remains difficult. Shoulder arthroplasty for this indication does not yield robust patient-reported outcomes and risks complications. Institutionally, we have found that aggressive treatment of the antecedent infection has helped optimize results from a reinfection and complication perspective. Any patient that initially presented with septic arthritis and a concern for osteomyelitis or were known to likely need a future arthroplasty were treated with a resection arthroplasty and antibiotic spacer early. Importantly, with the presented approach to this problem, there were no recurrent infections at the mid-term follow-up.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Cleeman E, Auerbach JD, Klingenstein GG, Flatow EL. Septic arthritis of the glenohumeral joint: a review of 23 cases. J Surg Orthop Adv. 2005;14(2):102–107. [PubMed] [Google Scholar]
- 2.Jeon IH, Choi CH, Seo JS, Seo KJ, Ko SH, Park JY. Arthroscopic management of septic arthritis of the shoulder joint. J Bone Joint Surg Am. 2006;88(8):1802–1806. doi: 10.2106/JBJS.E.00917. [DOI] [PubMed] [Google Scholar]
- 3.Klinger HM, Baums MH, Freche S, Nusselt T, Spahn G, Steckel H. Septic arthritis of the shoulder joint: an analysis of management and outcome. Acta Orthop Belg. 2010;76(5):598–603. [PubMed] [Google Scholar]
- 4.Mileti J, Sperling JW, Cofield RH. Shoulder arthroplasty for the treatment of postinfectious glenohumeral arthritis. J Bone Joint Surg Am. 2003;85(4):609–614. doi: 10.2106/00004623-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Dudkiewicz I, Salai M, Chechik A, Ganel A. Total hip arthroplasty after childhood septic hip in patients younger than 25 years of age. J Pediatr Orthop. 2000;20(5):585–587. doi: 10.1097/00004694-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Jerry GJ, Jr, Rand JA, Ilstrup D. Old sepsis prior to total knee arthroplasty. Clin Orthop Relat Res. 1988;(236):135–140. [PubMed] [Google Scholar]
- 7.Jupiter JB, Karchmer AW, Lowell JD, Harris WH. Total hip arthroplasty in the treatment of adult hips with current or quiescent sepsis. J Bone Joint Surg Am. 1981;63(2):194–200. [PubMed] [Google Scholar]
- 8.Kim YH. Total arthroplasty of the hip after childhood sepsis. J Bone Joint Surg Br. 1991;73(5):783–786. doi: 10.1302/0301-620X.73B5.1894666. [DOI] [PubMed] [Google Scholar]
- 9.Nazarian DG, de Jesus D, McGuigan F, Booth RE., Jr A two-stage approach to primary knee arthroplasty in the infected arthritic knee. J Arthroplasty. 2003;18(7 Suppl 1):16–21. doi: 10.1016/s0883-5403(03)00343-7. [DOI] [PubMed] [Google Scholar]
- 10.Chen CE, Wang JW, Juhn RJ. Total hip arthroplasty for primary septic arthritis of the hip in adults. Int Orthop. 2008;32(5):573–580. doi: 10.1007/s00264-007-0366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee GC, Pagnano MW, Hanssen AD. Total knee arthroplasty after prior bone or joint sepsis about the knee. Clin Orthop Relat Res. 2002;(404):226–231. doi: 10.1097/00003086-200211000-00036. [DOI] [PubMed] [Google Scholar]
- 12.Brook I, Frazier EH. Infections caused by Propionibacterium species. Rev Infect Dis. 1991;13(5):819–822. doi: 10.1093/clinids/13.5.819. [DOI] [PubMed] [Google Scholar]
- 13.Dodson CC, Craig EV, Cordasco FA, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg. 2010;19(2):303–307. doi: 10.1016/j.jse.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 14.Foruria AM, Fox TJ, Sperling JW, Cofield RH. Clinical meaning of unexpected positive cultures (UPC) in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(5):620–627. doi: 10.1016/j.jse.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Mook WR, Klement MR, Green CL, Hazen KC, Garrigues GE. The Incidence of propionibacterium acnes in open shoulder surgery: a controlled diagnostic study. J Bone Joint Surg Am. 2015;97(12):957–963. doi: 10.2106/JBJS.N.00784. [DOI] [PubMed] [Google Scholar]
- 16.Padegimas EM, Lawrence C, Narzikul AC, et al. Future surgery after revision shoulder arthroplasty: the impact of unexpected positive cultures. J Shoulder Elbow Surg. 2017;26(6):975–981. doi: 10.1016/j.jse.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Sethi PM, Sabetta JR, Stuek SJ, et al. Presence of Propionibacterium acnes in primary shoulder arthroscopy: results of aspiration and tissue cultures. J Shoulder Elbow Surg. 2015;24(5):796–803. doi: 10.1016/j.jse.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 18.Updegrove GF, Armstrong AD, Kim HM. Preoperative and intraoperative infection workup in apparently aseptic revision shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(3):491–500. doi: 10.1016/j.jse.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Morris BJ, Waggenspack WN, Jr, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Reverse shoulder arthroplasty for management of postinfectious arthropathy with rotator cuff deficiency. Orthopedics. 2015;38(8):e701–e707. doi: 10.3928/01477447-20150804-58. [DOI] [PubMed] [Google Scholar]
- 20.Schoch B, Allen B, Mileti J, Sperling JW, Cofield RH. Shoulder arthroplasty for the treatment of postinfectious glenohumeral arthritis. J Shoulder Elbow Surg. 2014;23(9):1327–1333. doi: 10.1016/j.jse.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115–1120. doi: 10.1016/j.jse.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249–2254. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 23.Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473(6):1860–1867. doi: 10.1007/s11999-015-4231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales: outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214–221. doi: 10.1177/03635465990270021701. [DOI] [PubMed] [Google Scholar]
- 26.Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587–594. doi: 10.1067/mse.2002.127096. [DOI] [PubMed] [Google Scholar]
- 27.Matsen FA, 3rd, Ziegler DW, DeBartolo SE. Patient self-assessment of health status and function in glenohumeral degenerative joint disease. J Shoulder Elbow Surg. 1995;4(5):345–351. doi: 10.1016/s1058-2746(95)80018-2. [DOI] [PubMed] [Google Scholar]
- 28.Padegimas EM, Narzikul A, Lawrence C, et al. Antibiotic spacers in shoulder arthroplasty: comparison of stemmed and stemless implants. Clin Orthop Surg. 2017;9(4):489–496. doi: 10.4055/cios.2017.9.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]