Fig. 1.
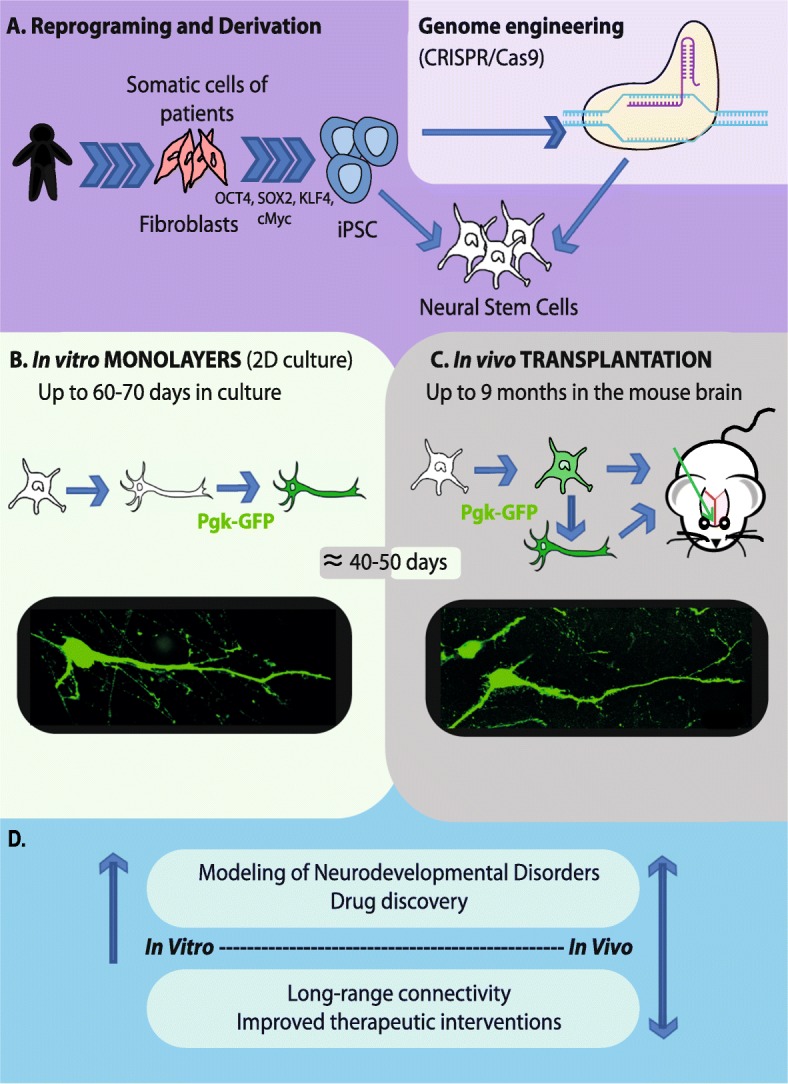
Main experimental designs for human iPSC models of monogenic neurodevelopmental disorders. a Patient’s specific iPSC are derived from fibroblasts using the four Yamanaka’s factors. Genome engineering using the CRISPR/Cas9 method allows the reversion of phenotypic defects by re-introducing the wild-type allele into the genome of iPSC lines. The CRISPR/Cas9 method also allows introduction of the mutation under study directly into the genome of control iPSC lines in order to compare visually similar phenotypes to those seen in the iPSC from patients. Both non-edited and isogenic iPSC are differentiated into the affected neuronal subtypes, mostly pyramidal cortical neurons in the case of cognitive disorders. b Viable neurons can be maintained in culture up to 70 days after the differentiation of neural stem cells (NSC). The transduction of neuronal cells with a green fluorescent protein (GFP)-lentivirus allows their visualization and phenotypic characterization using fluorescence microscopy. A GFP-labeled pyramidal neuron 40-45 days after the differentiation of NSC. c Neuronal precursors or neurons fully differentiated in vitro are transplanted into the brain of mouse neonates. The visualization of fluorescent neurons is done using fluorescent microscopy on brain slices. A transplanted GFP-labeled pyramidal neuron is illustrated at 40–50 days post-injection (picture from our experiments). Mice are maintained up to 9 months of age after grafting. d Comparative information and main therapeutics perspectives provided by the use of iPSC-derived neurons in vitro vs in vivo
