Abstract
Background:
Long-term mechanical ventilation in an Intensive Care Unit (ICU) exposes the patient to fungal colonization and invasive fungal disease due to the presence of indwelling catheters, administration of broad-spectrum antibiotics, and intravenous corticosteroids. A study is hence required to study the risk factors and incidence of fungal infection in these patients.
Methods:
A prospective observational study was carried out in the respiratory ICU of a tertiary care hospital for a period of approximately 1 year in which patients on mechanical ventilation (>7 days) were enrolled. Blood, urine, and endotracheal aspirate (ETA) of these patients were sent for fungal culture on day 1 and day 7 of mechanical ventilation. Fiberoptic bronchoscopy was done on day 7 and bronchoalveolar lavage along with transbronchial lung biopsy (TBLB) were sent for fungal culture.
Results:
During 7 days of ventilation, there was a statistically significant increase in the proportion of culture-positive ETA and urine samples. Overall, Candida albicans emerged as the most common colonizer. Blood candidemia was seen in 10% of patients on day 7 of mechanical ventilation. Fungal invasion of the lung, as evidenced by fungal culture-positive TBLB specimens, was seen in 17% of patients. Diabetes was found to be a statistically significant risk factor for respiratory and urinary tract colonization as well as invasive fungal disease.
Conclusion:
Long-term mechanical ventilation (>7 days) is strongly associated with fungal colonization of the respiratory tract and urinary tract. Appropriate prophylactic antifungals may be given and infection control practices to be observed to ensure minimum colonization and therefore infection in such settings.
Keywords: Fungal colonization, invasive fungal disease, mechanical ventilation, respiratory Intensive Care Unit
INTRODUCTION
Fungal infection has emerged as a significant health-care problem in recent years, owing to the extensive use of broad-spectrum antibiotics, long-term use of immunosuppressive agents, and increasing population of chronically ill patients with indwelling catheters.[1] Often, these risk factors overlap among patients admitted in the ICU leading to an increased rate of opportunistic fungal infections. Multiple site colonization with Candida spp. is also being commonly recognized as a major risk factor for invasive fungal infections in critically ill patients.[2]
The clinical features of fungal infection are nonspecific and pulmonary fungal infections often present radiologically as pneumonic shadows that can easily be confused with bacterial infection. This is a diagnostic challenge leading to a delay in starting antifungal therapy, thereby causing increased rates of mortality, especially in ICU setting.[3] There is limited data in Indian setting, hence present prospective study was undertaken to study fungal colonization and invasive fungal disease in long-term mechanically ventilated patients.
METHODS
The present prospective observational study was carried out in the respiratory ICU (RICU) of a tertiary care level respiratory institute from 2015 to 2016, in patients on long-term ventilation (>7 days). A total of 41 study participants were enrolled. On day 1 and day 7 of mechanical ventilation, urine, endotracheal aspirate (ETA), and blood of the patients were sent for fungal culture. Fiberoptic bronchoscopy (FOB) was also done on day 7 after due consent (received in only 33) and bronchoalveolar lavage (BAL) was sent for fungal culture. A FOB-guided transbronchial lung biopsy (TBLB) was planned only in those patients whose chest X-ray showed a new pneumonic patch during 7 days of mechanical ventilation. Transbronchial lung biopsies were guided by the location of the patch on the X-rays. However, consent for TBLB was received only in 28 patients.
In cases of pleural collection like effusion or empyema (found in 3/41 patients), the fluid was subjected to fungal culture. Ascitic fluid or cerebrospinal fluid (CSF) in suspected cases of meningitis along with pus pent up in any part of the body of the patient was also sent for fungal culture.
Microbiological investigations included microscopy and culture. On direct microscopy, samples were examined by preparing wet mount using 10% potassium hydroxide and/or Gram stain (except for blood) to detect the presence of yeast cells, yeast with pseudohyphae, or septate hyphae for the mold. The samples for fungal culture were inoculated on Sabouraud's dextrose agar with and without antibiotic and incubated at 25°C and 37°C. Yeast was further identified by germ tube test, corn meal agar test, chrome agar assay, and antibiotics. The molds were identified using lactophenol cotton blue mount (LPCB) and slide culture.[4]
Finally, Candida score was calculated for all patients and its predictive value for invasive candidiasis was estimated by comparing with diagnosed cases of invasive candidiasis (by either blood fungal culture or bronchial biopsy culture or ascitic/pleural/CSF culture).[5]
Statistical analysis was done using SPSS version 17 (IBM, New York, USA). The statistical tests which have been used in the study were Chi-square test, Fisher's exact test, and z proportion test.
RESULTS
Out of a total of 41 patients in our study, it was observed that maximum patients belonged to age group ≥60 years (20/41, 48%), followed by 45–59 years (19/41, 46%) and age <45 years (2/41, 4.8%). Thirty-one were male (31/41, 76%) and the rest ten were female (10/4124%).
The most common diagnosis was that of pulmonary tuberculosis sequelae with or without chronic obstructive pulmonary diseases (COPD), seen in 17/41 (41%) patients; followed by COPD seen in 13 (32%) patients, pneumonia in 6/41 (5%) patients, and interstitial lung disease in 2/41 (5%) patients.
Prior long-term steroid intake (>3 times in the past 6 months) was the most common risk factor present in 35/41 (81%), followed by diabetes mellitus in 12/41 patients (29%) and fibrocavitatory disease in chest X-ray in 8 (19%) patients. Presence of other risk factors were looked into such as bronchogenic carcinoma receiving chemotherapy, intake of immunosuppressive drugs other than steroids and HIV coinfection. However none of our enrolled patients had these risk factors.
The fungi isolated in various clinical samples between day 1 and day 7 of intubation are summarized in Table 1.
Table 1.
Fungal culture pattern on day 1 and day 7 of mechanical ventilation
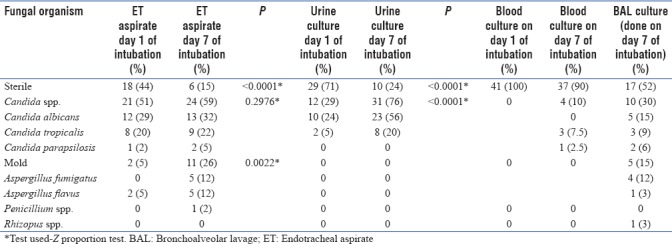
From the table, we can see that there is a rise in airway colonization by fungus (both Candida spp. and Aspergillus spp.) from day 1 to day 7. However, this increase was found to be statistically significant in case of Aspergillus spp. (P = 0.0022) as seen in ET (Endotracheal aspirate) cultures.
Urine colonization was found only with Candida spp., which increased from day 1 to day 7 (P < 0.0001).
Pleural fluid sent for fungal culture from patients with associated pleural diseases did not yield a fungal growth. None of our patients had meningitis or pus collection anywhere in the body.
Out of the 4 positive blood cultures on day 7 of intubation, three yielded Candida tropicalis and one Candida parapsilosis. Fungal culture of TBLB was positive in 5/28 (17%) patients. Out of them, 1 sample yielded C. tropicalis and 4 yielded Aspergillus fumigatus. The patient whose TBLB specimen yielded C. tropicalis also had blood culture positive for C. tropicalis. Thus, invasive candidiasis was diagnosed in 4/41 (9.75%) patients who yielded 4 positive blood cultures and 1 positive TBLB fungal culture. Diagnosis of invasive aspergillosis was made in 4 patients. Hence, in total, 8/41 patients were diagnosed with invasive fungal disease (either bloodborne or pulmonary).
Of all the risk factors investigated in our study, only diabetes mellitus had a statistically significant relationship with fungal colonization as evidenced by culture status change (either change of culture status from sterile to nonsterile between 7 days or change in the colonizing organism) in ET aspirate (P = 0.038) and urine samples (P = 0.03) between day 1 and day 7 of mechanical ventilation (test used – Fischer's exact test). Out of the 12 diabetic patients on day 1 of intubation, fungi isolated in their ET aspirate samples were Candida albicans and C. tropicalis (both 3/12,25%), followed by Aspergillus spp. (1/12,8%). On day 7 of intubation, among ET aspirates, the number of culture samples positive for Aspergillus spp. increased (6/12,50%) with C. albicans seen in 25% of samples (3/12), followed by C. tropicalis (1/12,8%) and Penicillium (1/12,8%). Out of the 12 diabetic patients on day 1 of intubation, 33% of all the urine samples (4/12) were positive for C. albicans growth. Rest were sterile (66%). On day 7 of intubation, 75% (9/12) of the urine samples were positive for C. albicans growth and the rest 25% (3/12) were positive for C. tropicalis growth. All the four patients whose TBLB yielded Aspergillus growth were diabetic patients. Thus, diabetes emerged as a risk factor for invasive aspergillosis (P = 0.005), Fischers exact test was used. Out of the 4 patients with positive blood fungal culture, 2 patients had diabetes. Hence, out of the 8 patients in total diagnosed with invasive fungal disease (either bloodborne or pulmonary), 6 came out to be diabetic patients. Again diabetes emerged as a risk factor for invasive fungal disease (P = 0.0042, Fischer's exact test used).
Candida score was calculated for all patients. Out of the 4 patients diagnosed with invasive candidiasis, all 4 had Candida score = 3 (presence of both severe sepsis and multifocal candidial colonization). Out of the remaining 24 patients (as the criteria for fungal invasion evidenced by blood and TBLB fungal culture positivity was only fulfilled in 28 patients as in other cases consent for TBLB was not received), 17 patients had Candida score <3. Relationship between Candida score ≥3 and invasive candidiasis also came out to be statistically significant (P = 0.0161, Fischer's exact test used).
DISCUSSION
In this study, we evaluated the rates of fungal colonization and invasive fungal disease in long-term mechanically ventilated patients, the majority of whom were posttubercular sequelae patients, followed by patients of COPD suffering from respiratory failure and/or sepsis.
There was statistically significant increase in fungal colonization rates in ETA samples between day 1 and day 7 of intubation. Similar finding was seen by Charles et al.who had studied the weekly increments in colonization rates of ETA in critically ill study patients till their death/discharge and found that to be significant.[6] He identified various risk factors which lead to an increase in candidial colonization such as exposure to broad-spectrum antibiotics and time of ICU admission. These were also present in majority of our study patients. However, much higher proportion of C. tropicalis colonization in our study was seen as compared to studies done outside India.[7,8] The study by Kothavade et al. also shows that there is rising trend of increased rates of Candidia spp. infections in Indian hospitals caused by members of the non-albicans group, of which Candida tropicalis is the most dominant member, probably due to its greater biofilm forming capacity.[9,10]
In urine fungal culture, there was significant increase in colonization rates between days 1 and 7 of intubation, with C. albicans being the most common. However, keeping with the increased rates of nonalbicans isolation in Indian hospitals, C. tropicalis was isolated at a much higher rate than in studies done outside India.[7,11]
Candida spp. usually are commensals of skin which colonize mucosal surfaces usually in immunosuppressed, whereas Aspergillus spp. colonize after being transmitted as spores through air.[12,13] This is attested by our study in which there is significant increase in mold proportions in ETA fungal cultures (P = 0.0022), indicating possible air-borne route of transmission of fungal spores in ICU atmosphere.
The sterile blood cultures at ICU admission showed fungal growth on day 7 in 4 patients. It is seen in a study by Das et al. that the most common predisposing factors for blood candidemia are the use of broad-spectrum antibiotics, the presence of an intravascular device, admission to an Intensive Care Unit (ICU), and recent surgery.[14] Other than recent surgery all the other risk factors were present in our patients whose blood culture yielded Candida spp.
There was a single patient in our study whose blood culture and TBLB fungal culture both yielded C. tropicalis. The positive TBLB fungal culture in this patient could be explained by the fact that he also had blood candidemia and that pulmonary invasion occurred secondary to hematogenous dissemination. However, there is another possibility. Invasive candidiasis actually encompasses three distinct entities (a) candidemia with deep-seated infection, (b) candidemia without deep-seated infection, and (c) deep-seated infection without candidemia.[15] In a study done by Leroy et al. in 2009, it was found that out of the 300 patients enrolled with invasive candidiasis, around 40% had isolated candidemia, while around 32% had invasive candidiasis without candidemia and 28% had both candidemia and invasive candidiasis.[16] However, there was no statistically significant difference in mortality between the groups. It appears that candidemia with concomitant invasive candidiasis can be a distinct clinical entity and further studies are required to look into its effects on patient mortality.
Diabetes mellitus emerged as a risk factor in our study which is a known risk factor for aspergillosis.[17] Interestingly, when we looked at the BAL fluid and ETA culture isolates on day 7 of intubation in people with diabetes, Aspergillus spp. emerged as the dominant colonizer (50% in ETA samples and 43% in BAL samples). This is in contrast to C. albicans which was overall the most common colonizer. Further studies with larger sample size are required to substantiate this finding.
50% of blood culture-positive patients in our study had diabetes, which is an established risk factor for blood-borne candidemia.[18]
Other risk factors that we studied were steroid therapy and fibrocavitatory disease, both of which are known risk factors for fungal colonization.[19] However, in our study, no significant relationship was found, which may be due to small number of patients with fibrocavitatory disease (n = 8) and a number of confounding factors such as steroid therapy during admission (most of the patients had exacerbations of chronic respiratory diseases), broad-spectrum antibiotics usage, and mechanical ventilation administered to all the patients along with indwelling catheters and intravenous lines.
Candida score is frequently used to decide to start antifungal therapy in nonneutropenic severely ill patients which consist of parameters such as multifocal candidial colonization, surgery, total parenteral nutrition, and severe sepsis.[20] We used it to stratify our patients into high-risk groups with likelihood of invasive candidial infections and start antifungal therapy in them and subsequently decreasing mortality and morbidity.
Our study had limitation that sample size was small and TBLB could only be done in 28 patients. TBLB done on day 7 of mechanical ventilation could have helped us to conclusively identify cases of invasive fungal involvement of the lung. In addition, due to the unavailability of HRCT scans on day 7 of ventilation, segmental localization of the pneumonic patch could not be done which may have decreased our TBLB yield. Finally, since ours was a RICU, samples from extrapulmonary sites were lacking.
CONCLUSION
In the patients on long-term mechanical ventilation in the ICU, many factors coexist to make them prone for fungal colonization. These factors may be broad-spectrum antibiotics, intravenous steroids, urinary catheterization, presence of peripheral or central venous catheter, and presence of sepsis. These factors interplay with various patient-specific risk factors such as diabetes mellitus, prior oral steroid intake and presence of fibrocavitatory disease, and increase the risk of fungal colonization or invasive fungal disease. Treating physicians working in an ICU and especially in RICU should be aware of the risk of fungal colonization and its progression to invasive or disseminated fungal disease in patients on long-term mechanical ventilation admitted under their care. Preliminary data in the present study warrant replication in the form of a larger study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Samonis G, Gikas A, Toloudis P, Maraki S, Vrentzos G, Tselentis Y, et al. Prospective study of the impact of broad-spectrum antibiotics on the yeast flora of the human gut. Eur J Clin Microbiol Infect Dis. 1994;13:665–7. doi: 10.1007/BF01973996. [DOI] [PubMed] [Google Scholar]
- 2.Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3:685–702. doi: 10.1016/s1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 3.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin Infect Dis. 2006;43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 4.Forbes BA, Sahm DF, Weissfeld AS. Bailey and Scott's Diagnostic Microbiology. 11th ed. St. Louis: Mosby, Inc.; 2002. pp. 711–97. [Google Scholar]
- 5.León C, Ruiz-Santana S, Saavedra P, Galván B, Blanco A, Castro C, et al. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: A prospective multicenter study. Crit Care Med. 2009;37:1624–33. doi: 10.1097/CCM.0b013e31819daa14. [DOI] [PubMed] [Google Scholar]
- 6.Charles PE, Dalle F, Aube H, Doise JM, Quenot JP, Aho LS, et al. Candida spp. Colonization significance in critically ill medical patients: A prospective study. Intensive Care Med. 2005;31:393–400. doi: 10.1007/s00134-005-2571-y. [DOI] [PubMed] [Google Scholar]
- 7.León C, Alvarez-Lerma F, Ruiz-Santana S, León MA, Nolla J, Jordá R, et al. Fungal colonization and/or infection in non-neutropenic critically ill patients: Results of the EPCAN observational study. Eur J Clin Microbiol Infect Dis. 2009;28:233–42. doi: 10.1007/s10096-008-0618-z. [DOI] [PubMed] [Google Scholar]
- 8.Azoulay E, Timsit JF, Tafflet M, de Lassence A, Darmon M, Zahar JR, et al. Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest. 2006;129:110–7. doi: 10.1378/chest.129.1.110. [DOI] [PubMed] [Google Scholar]
- 9.Kothavade RJ, Kura MM, Valand AG, Panthaki MH. Candida tropicalis: Its prevalence, pathogenicity and increasing resistance to fluconazole. J Med Microbiol. 2010;59:873–80. doi: 10.1099/jmm.0.013227-0. [DOI] [PubMed] [Google Scholar]
- 10.Kaur R, Goyal R, Dhakad MS, Bhalla P, Kumar R. Epidemiology and virulence determinants including biofilm profile of Candida infections in an ICU in a tertiary hospital in India. J Mycol. 2014:303491. [Google Scholar]
- 11.CandiRea Study Group. Bougnoux ME, Kac G, Aegerter P, d'Enfert C, Fagon JY, et al. Candidemia and candiduria in critically ill patients admitted to Intensive Care Units in France: Incidence, molecular diversity, management and outcome. Intensive Care Med. 2008;34:292–9. doi: 10.1007/s00134-007-0865-y. [DOI] [PubMed] [Google Scholar]
- 12.Sardi JC, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJ. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62:10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 13.Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: A clinical review. Eur Respir Rev. 2011;20:156–74. doi: 10.1183/09059180.00001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das I, Nightingale P, Patel M, Jumaa P. Epidemiology, clinical characteristics, and outcome of candidemia: Experience in a tertiary referral center in the UK. Int J Infect Dis. 2011;15:e759–63. doi: 10.1016/j.ijid.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284–92. doi: 10.1093/cid/cit006. [DOI] [PubMed] [Google Scholar]
- 16.Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, et al. Epidemiology, management, and risk factors for death of invasive candida infections in critical care: A multicenter, prospective, observational study in France (2005-2006) Crit Care Med. 2009;37:1612–8. doi: 10.1097/CCM.0b013e31819efac0. [DOI] [PubMed] [Google Scholar]
- 17.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70:270–7. doi: 10.1136/thoraxjnl-2014-206291. [DOI] [PubMed] [Google Scholar]
- 18.Muskett H, Shahin J, Eyres G, Harvey S, Rowan K, Harrison D, et al. Risk factors for invasive fungal disease in critically ill adult patients: A systematic review. Crit Care. 2011;15:R287. doi: 10.1186/cc10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswas D, Agarwal S, Sindhwani G, Rawat J. Fungal colonization in patients with chronic respiratory diseases from Himalayan region of India. Ann Clin Microbiol Antimicrob. 2010;9:28. doi: 10.1186/1476-0711-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.León C, Ruiz-Santana S, Saavedra P, Galván B, Blanco A, Castro C, et al. Usefulness of the “Candida score” for discriminating between candida colonization and invasive candidiasis in non-neutropenic critically ill patients: A prospective multicenter study. Crit Care Med. 2009;37:1624–33. doi: 10.1097/CCM.0b013e31819daa14. [DOI] [PubMed] [Google Scholar]


