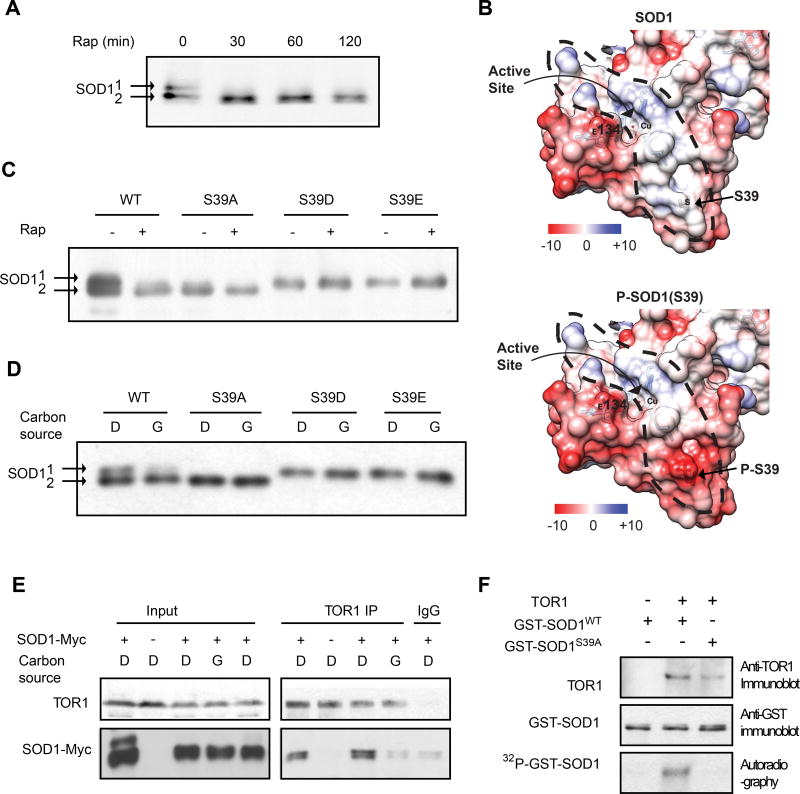
Figure 3. TORC1 interacts with SOD1 and phosphorylates SOD1 at S39 in yeast.
(A) Rapamycin induces electrophoretic shift of SOD1 protein. Yeast cells expressing SOD1-Myc9 were treated with 100 nM rapamycin for different times. The electrophoretic mobility of SOD1 was analyzed by immunoblot with a Myc-specific antibody.
(B) S39 is located at the entry of a positively charged tunnel (circled by a dotted line) that guides the inflow of negatively charged O2− substrates to the active site. Shown are 3D crystal structures of yeast SOD1 with electrostatic surface with S39 in unphosphorylated (top) or modeled phosphorylated state (bottom). Blue, electropositive charge; White, neural; Red, electronegative charge; Electrostatic potential, kcal mol−1 e−1.
(C) S39 is phosphorylated in a rapamycin-sensitive manner. Yeast cells expressing WT or mutant SOD1-Myc9 were treated with 100 nM rapamycin for 30 min. The electrophoretic mobility of SOD1-Myc9 was analyzed by immunoblot with a Myc-specific antibody.
(D) S39 phosphorylation is regulated by nutrients. Yeast cells expressing WT or S39 mutant SOD1-Myc9 proteins were shifted from glucose (D, dextrose) to glycerol medium (G) for 3 hrs. Electrophoretic mobility of SOD1-Myc9 was immunoblot with a Myc-specific antibody.
(E) TORC1 interacts with SOD1 in a nutrient-dependent manner. Yeast cells expressing SOD1-Myc9 were shifted from glucose (D, dextrose) to glucose or glycerol (G) medium for 3 hr. TORC1 was immunoprecipitated with a TOR1 specific antibody. TORC1 interaction with SOD1-Myc9 was analyzed by immunoblot.
(F) TORC1 phosphorylates SOD1 at S39. Immunoprecipitated TORC1 from cells cultured in glucose medium was incubated with bacterially produced recombinant GST-SOD1WT or GST-Sod1S39A in the presence of γ-[32P]-ATP. Phosphorylation of GST-SOD1 proteins was detected by autoradiography.
See also Figures S1 and S2; Table S1.