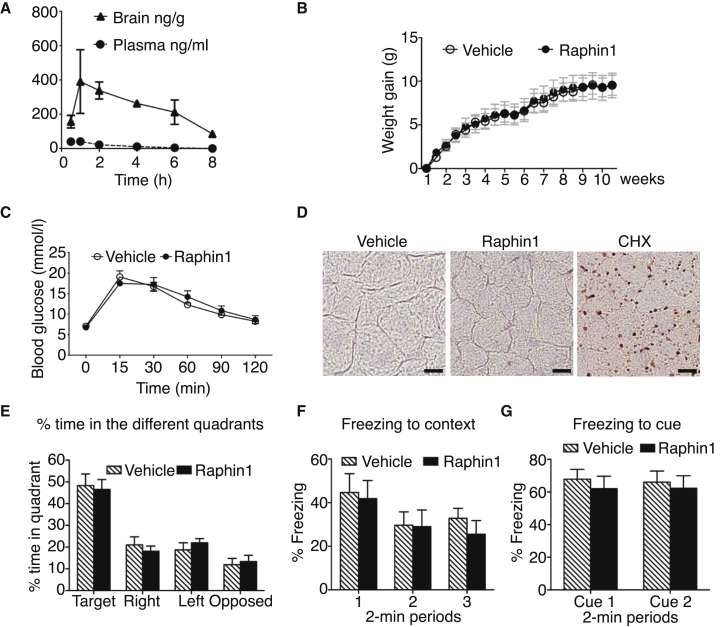
Figure 6.
No Adverse Effects of Raphin1 Treatment on Body Weight Gain, Pancreatic and Liver Function, or Memory in Mice
(A) Concentration of Raphin1 in the brain and plasma at the indicated time after a single oral administration of Raphin1 at 2 mg/kg. Data are means ± SEM; n = 3.
(B) Total body weight gain of wild-type mice treated with Raphin1 at 2 mg/kg or vehicle for 10 weeks. Data are means ± SEM; n = 10 per group.
(C) Glucose tolerance test on wild-type mice after treatment with Raphin1 at 2 mg/kg or vehicle for 8 weeks. Data are means ± SEM; n = 8 (vehicle) and n = 9 (Raphin1).
(D) Oil Red O staining of liver in wild-type mice treated with Raphin1 or CHX at 40 mg/kg or vehicle. Scale bar, 10 μm.
(E) Quadrant occupancy after training and removal of the platform in the Morris water maze of wild-type mice treated with Raphin1 at 2 mg/kg or vehicle for 2 weeks. Data are means ± SEM, n = 9 (vehicle) and n = 10 (Raphin1).
(F and G) Response to fear conditioning—freezing to context (F) or freezing to cue (G)—of wild-type mice treated with Raphin1 at 2 mg/kg or vehicle for 3 weeks. Data are means ± SEM; n = 10 per group.
There were no significant differences between Raphin1- and vehicle-treated mice as revealed by an unpaired two-tailed Student’s t test (B, C, E, F, and G).
See also Figures S5 and S6 and Videos S1, S2, and S3.