Fig. 1 |. Chaperome connectivity from normal cellular states to conditions characterized by increasing stress.
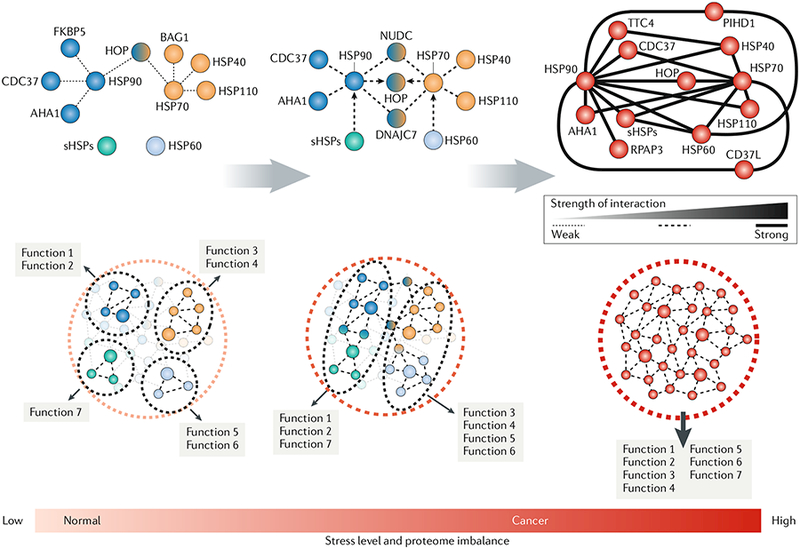
Under normal cellular conditions, the chaperome acts as a flexible and versatile connector of cellular pathways. Such flexibility is enabled by dynamic interactions among the chaperome members and between the major chaperome machineries. In these conditions, each chaperome machinery executes its set of dedicated functions with the use of individual sets of chaperones and co-chaperones. Such flexibility also permits a rapid chaperome rewiring when cells are exposed to acute stress (for example, heat) and certain chronic stresses to enable cellular stability and functionality. Networks are context-dependent, and the formation, re-wiring or restructuring of connectivities all depend on the applied molecular stress. Cellular stress increases the connectivity between distinct chaperome machineries, with the goal of increasing their functional diversity and competence. Certain stresses, such as MYC hyperactivation during oncogenesis (see the main text), can lead to a state of chaperome hyperconnectivity, where cellular demand requires maximal chaperome participation. Here, hyperconnectivity of the chaperome is executed by an increase in affinity between the chaperome machineries through their associated chaperones and co-chaperones. Thus, dynamic interactions between the chaperome members are characteristic of normal physiological conditions, but cellular stress alters the thermodynamics of such interactions, resulting in increased stability of chaperome complexes. Such a change also produces an altered functional interdependence among chaperome members. Most of the current data relates to the heat shock protein 90 (HSP90)-HSP70 chaperome, which we present as an example here, but the chaperome extends beyond these highly studied members. AHA1, activator of HSP90 ATPase 1; BAG1, BAG family molecular chaperone regulator 1; CD37L, HSP90 co-chaperone CDC37-ίike 1; CDC37, cell division cycle 37; FKBP5, FK-506-binding protein 5; HOP, HSP70-HSP90 organizing protein; NUDC, nuclear distribution protein C; PIHD1, PIH1 domain-containing protein 1; RPAP3, RNA polymerase Il-associated protein 3; sHSPs, small HSPs; TTC4, tetratricopeptide repeat domain 4.
