Fig. 2 |. Functional gains from formation of multimeric chaperome scaffolding platforms under cellular stress.
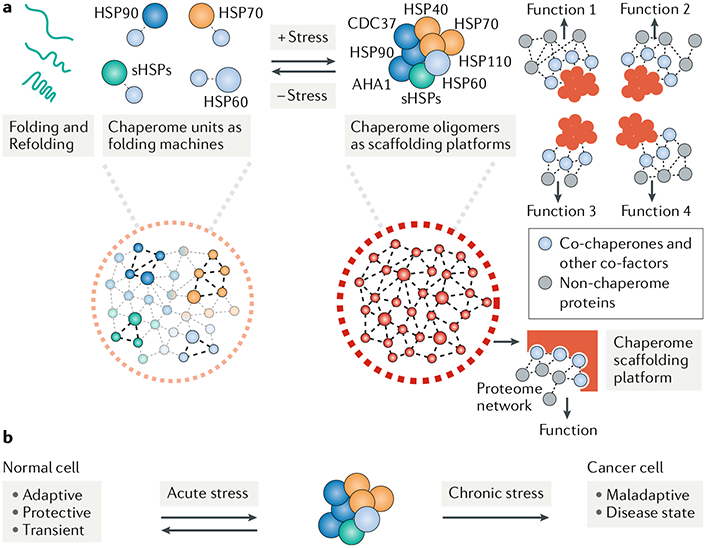
a | Cellular stress results in the formation of multimeric chaperome complexes of increased stability These structures, which we call chaperome scaffolding platforms, form after stress to execute folding-independent functions and provide a template for the acquisition of new activities. Chaperome scaffolding platforms have the advantage of increased quaternary structure diversity, where formation of new connections between chaperome units may increase the function and versatility of such platforms35,43. Stabilization of chaperome platforms by reducing the dynamic nature of chaperome-chaperome interactions may improve efficiency. These high-affinity chaperome complexes may help organize and maintain the function of the oncoprotein complement of a cancer cell in a tumour-specific and transformation-specific manner. In some cases, these context-dependent functions include the activation of signalling pathways in certain leukaemias73, the processing of key mRNAs of lymphoma subtypes78 and the maintenance of the viral oncoproteome in gammaherpesvirus-associated malignancies74. b | Formation of the chaperome platforms enables a dynamic adaptation to acute insults, providing a protective mechanism for cells under transient stress. When stresses become chronic, the adaptive functions meant to provide survival under acute stress become cemented and lead to maladaptive phenotypes associated with disease. AHA1, activator of HSP90 ATPase 1; CDC37, cell division cycle 37; HSP, heat shock protein; sHSPs, small HSPs.
