Figure 1. Macrolides narrow the nascent peptide exit tunnel but allow synthesis of certain proteins.
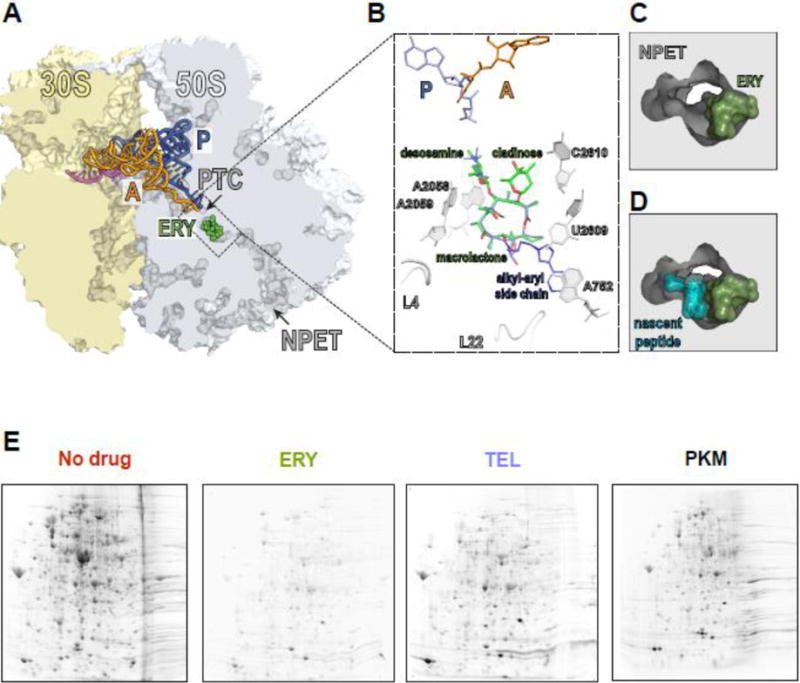
(A) The macrolide binding site in the bacterial ribosome. A cross-cut of the ribosome showing the A- and P-site tRNAs (orange and blue, respectively) and a segment of mRNA (magenta). ERY (green) and all the other macrolides bind in the NPET at a short distance from the PTC.
(B) The macrolide binding site is composed primarily of rRNA. The macrolactone ring of different macrolides (for comparison, the structures of ERY, green, and TEL, blue, have been superimposed) lays flat against the NPET wall and the C3 and C5 sugars protrude towards the PTC but do not reach its active site. C5 desosamine interacts with the splayed-out A2058 and A2059 rRNA residues in the NPET. C2610 nucleotide contacts C3 cladinose (present in ERY, but lacking in TEL). The alkyl-aryl side chain of ketolides, such as TEL, usually extends away from the PTC: In E. coli, it interacts with the A752-U2609 base pair, but its placement may differ in other bacteria [74, Dunkle, 2010 #7182, 75]. The loops of proteins L4 and L22, which form a constriction in the NPET, may directly interact with the side chains of some macrolides [76].
(C) and (D) View into the NPET from the PTC showing that the macrolide (ERY) narrows the tunnel’s aperture (C). The remaining opening of the NPET is nevertheless wide enough for a nascent peptide to be threaded through (D).
(E) Specific proteins are synthesized in macrolide-treated cells. Gel electrophoresis analysis of radiolabeled proteins translated in E. coli cells in the absence of antibiotics (No drug), or exposed to high concentrations of ERY, semi-synthetic ketolide TEL, or the natural ketolide pikromycin (PKM).
