Figure 3. Macrolides are selective modulators of the peptidyl transferase center.
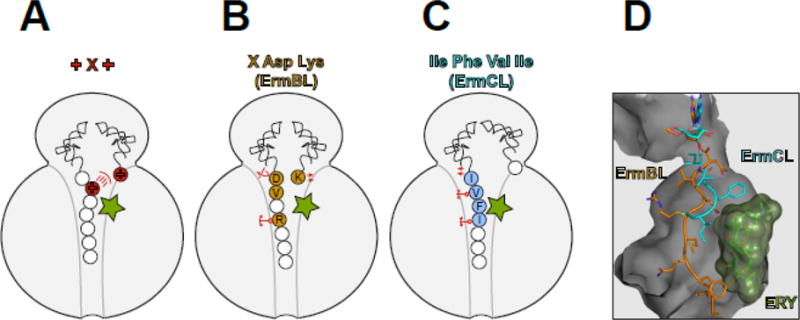
The interplay between the macrolide molecule and a MAM-containing nascent peptide alters the PTC properties and inhibits peptide bond formation. In (A–C), the residues critical for stalling are colored and in (B) and (C) are indicated with the single-letter code. (A) Stalling at the +X+ MAM may occur because the macrolide orients the lengthy, positively charged side chain of the penultimate amino acid of the nascent peptide towards the PTC A site, preventing the accommodation of the similarly long and positively charged acceptor amino acid. (B) The macrolide imposes an unfavorable orientation of the C-terminal Asp residue of the ErmBL peptide that contains the X-Asp-Lys MAM [19, 21]. The placement of the acceptor Lys in the A site is also suboptimal. The orientation of several key PTC nucleotides is altered in the stalled ribosome. The mobility of the nascent peptide in the NPET is restricted due to the antibiotic presence and specific interactions of the Arg residue of the nascent chain with rRNA of the tunnel wall. (C) Interactions of the ErmCL nascent chain with the NPET nucleotides and antibiotic misplace the peptide’s C-terminal residue in the PTC [20]. The adverse conformation of the PTC nucleotides prevents accommodation of the A site amino acid [20, 72]. (D). The different placement of the ErmBL and ErmCL nascent peptides in the NPET of the ERY-stalled ribosome shows that the peptide trajectory depends on the amino acid sequence.
