Figure 4. The general model of macrolide action upon protein synthesis.
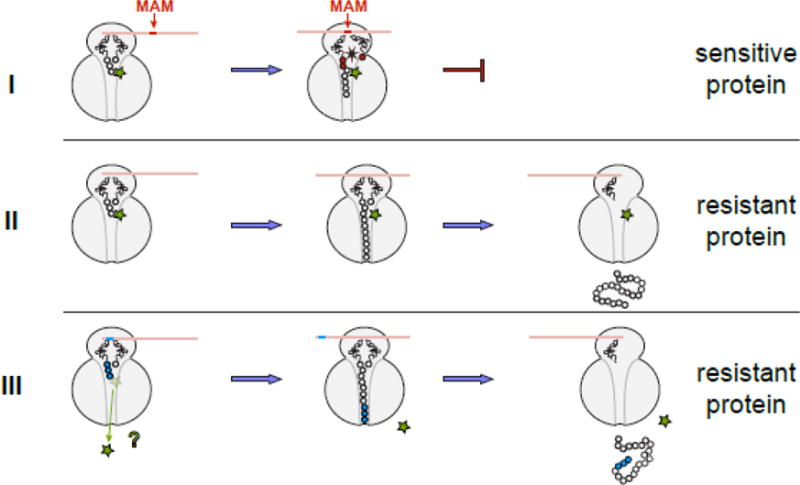
Translation of any protein can be initiated by the macrolide-bound ribosome and the N-termini of the majority of polypeptides can be threaded through the drug-obstructed NPET. The subsequent fate of the protein being made depends on its sequence and the structure of the bound antibiotic. (I) Translation of sensitive proteins is interrupted because the drug-bound ribosome is unable to efficiently catalyze peptide bond formation during synthesis of the MAM sequence. The structure of the macrolide molecule bound in the NPET dictates the spectrum of the MAM sequences and therefore, defines which proteins will be inhibited. If translation is arrested close to the start of the ORF, when the nascent peptide is short (<10 amino acids), peptidyl-tRNA likely dissociates from the ribosome (the effect known as peptidyl-tRNA drop-off) [7, 8, 77]. (II) If the protein sequence lacks MAMs, its translation will proceed unimpeded and the full-size protein will be produced in the cell exposed to the antibiotic. (III) Hypothetically, some proteins might be able to dislodge the antibiotic from the ribosome at the early stages of translation; in this case, the drug-free ribosome completes the synthesis of such protein. Antibiotic eviction has been observed with some artificial short peptides [13–16], but has not been demonstrated yet for the cellular polypeptides; yet this scenario remains a possibility for at least some of the bacterial proteins.
