Figure 6. Nascent peptides turn the ribosome into a small molecule sensor.
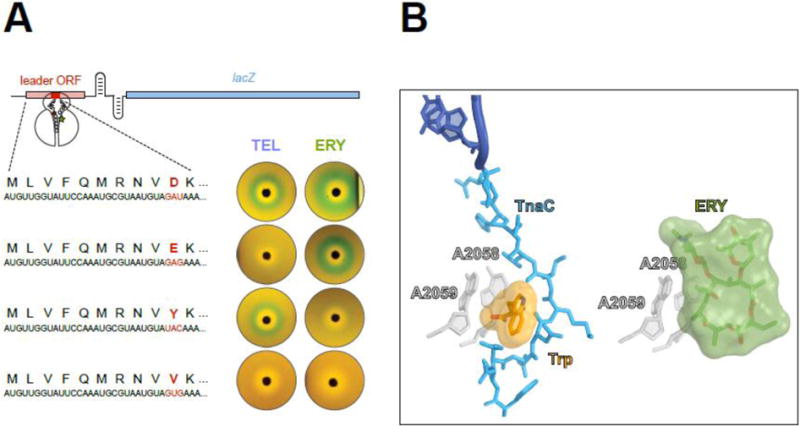
(A) Single amino acid changes in the ErmBL nascent peptide alters the ribosomal response to structurally different macrolides. The reporter cassette mimics an inducible erm resistance operon (see Figure 5), in which the resistance gene was replaced with lacZ. The reporter induction is visualized by the green color of the cell lawn in the vicinity of an antibiotic-containing disk placed on the agar plate. Both ketolide TEL or cladinose-containing ERY arrest ermBL translation and activate the reporter when the 10 th codon (red) of ermBL specifies Asp (wild type). Changing th the 10 codon to Glu preserves the response to ERY but eliminates the response to TEL. Tyr-10 allows the ribosome to respond exclusively to ketolides. Val-10 precludes the response to either TEL or ERY.
(B) The TnaC nascent peptide allows sensing of tryptophan. Activation of the tna operon depends on ribosome stalling on the tnaC leader ORF [60, 61]. Structural studies of the TnaC-stalled ribosome suggest that one of the two observed tryptophan molecules binds at the A2058/A2059 crevice (left image) [63], the same site that is exploited by macrolide antibiotics for binding to the ribosome (right image) [78]. Similar to the coordinated action of macrolides and nascent peptide in inducing translation arrest, the TnaC peptide cooperates with tryptophan to disrupt the PTC function and stall the ribosome at the last sense codon of the tnaC ORF.
