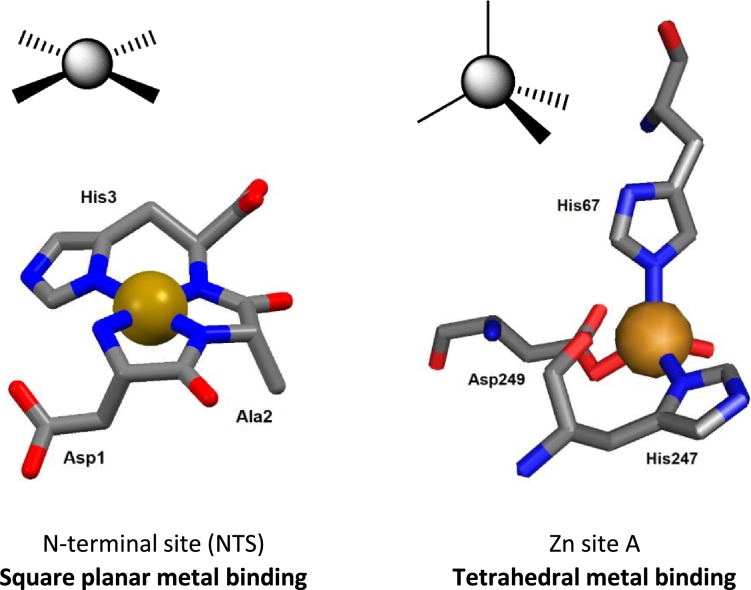
Fig. 2.
Contrasting geometries of metal binding sites on albumin. Left: square planar coordination of Cu2+ or Ni2+ at the NTS site; the structure shown is derived from molecular modelling. The N-terminal amino group, two deprotonated backbone amide N atoms and the N(delta) of the imidazole ring of His3 form a square plane around the central metal ion. Right: tetrahedral coordination of Zn2+ at site A in human serum albumin (pdb 5ijf). His67 uses its N(epsilon) N atom, whilst His247 binds via N(delta). Asp249 binds in mono-dentate fashion, with the second carboxylate O at ca. 2.6 Å distance, too long for a metal-ligand bond. Typically for zinc sites in proteins, angles between ligands deviate substantially from the ideal tetrahedral angle (109.5°) and vary between 95° and 125°. Metal ions are rendered in gold, N atoms in blue, O atoms in red, carbon atoms in grey. No H atoms are shown.