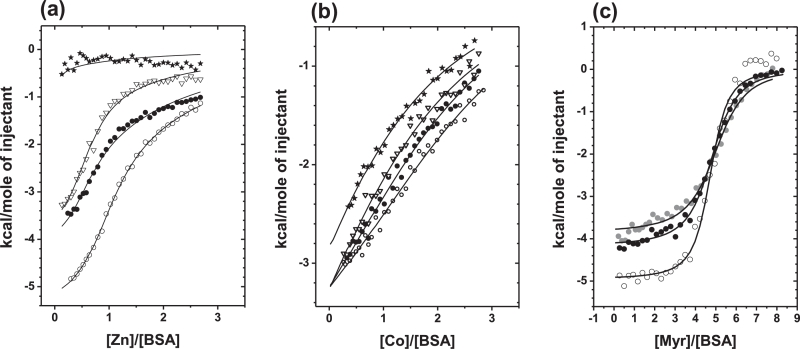
Fig. 5.
Isothermal titration calorimetry experiments demonstrate the mutual modulation of metal and fatty acid binding to bovine albumin. The presence of the C14:0 fatty acid myristate (○, 0 mol. equiv.; ●, 1 mol. equiv.; ▽, 3 mol. equiv.; and ★ 5 mol. equiv.) affects the binding capacity of albumin for Zn2+ (a) and Co2+ (b) under near-physiological conditions (pH 7.4, 50 mM Tris-Cl, 50 mM NaCl). Co2+ binding to albumin is not only weaker than that of Zn2+, but the effect of FFAs on Zn2+ binding is also much more pronounced than that of Co2+. (c) The presence of 1 mol. equiv. of Zn2+ ( ) or Co2+ (●) affects the energetics of fatty acid binding relative to the metal-free experiment (○), likely due to the need to remove the metal before the FFA can bind. Notably, again the effect for Zn2+ is larger than that for Co2+.
) or Co2+ (●) affects the energetics of fatty acid binding relative to the metal-free experiment (○), likely due to the need to remove the metal before the FFA can bind. Notably, again the effect for Zn2+ is larger than that for Co2+.