I read with interest the study by De Archangelis et al 1 on the protective role of hemidesmosomes against colitis and colorectal cancer using genetically modified mouse integrin α6 subunit mutant models. I was however surprised to read that, based on their observations with these α6 mutant mice, the authors concluded that the α6β4 integrin can be classified as a tumour suppressor in the colon. Indeed, earlier studies have reported that in carcinomas, α6β4 can be released from hemidesmosomes to become associated with microfilament-associated cell motility adhesomes and, consequently, engage in various signal transduction pathways that contribute to tumour progression.2 3 While it is recognised that the roles of α6β4 may be dependent on the tissue-context as underlined by the authors, it remains increasingly evident that the alternative messenger RNA splicing of the α6 subunit constitutes a key-contributing factor for the definition of the function of α6β4 in determining the fate of cancer cells,4 including colorectal cancer cells.5
First, it is noteworthy that both α6 subunits are normally expressed in the intestinal epithelium but in distinct compartments, α6A being found in the proliferative cells of the glands in both the small and large intestine while α6B is restricted to the quiescent/differentiated cells located on the villus and surface epithelium (figure 1).5 6 Second, the expression of the α6β4 receptor is increased in colorectal cancer cells5 7 and it is in fact its pro-proliferative α6Aβ4 form that is found to be largely expressed under this context,8 whereas its α6Bβ4 counterpart appears to exert antiproliferative influences.5 Altogether, these data indicate that α6β4 performs distinct functions in intestinal and colonic cells according to the specific α6 (A or B) splicing variant that constitutes the heterodimeric receptor.
Figure 1.
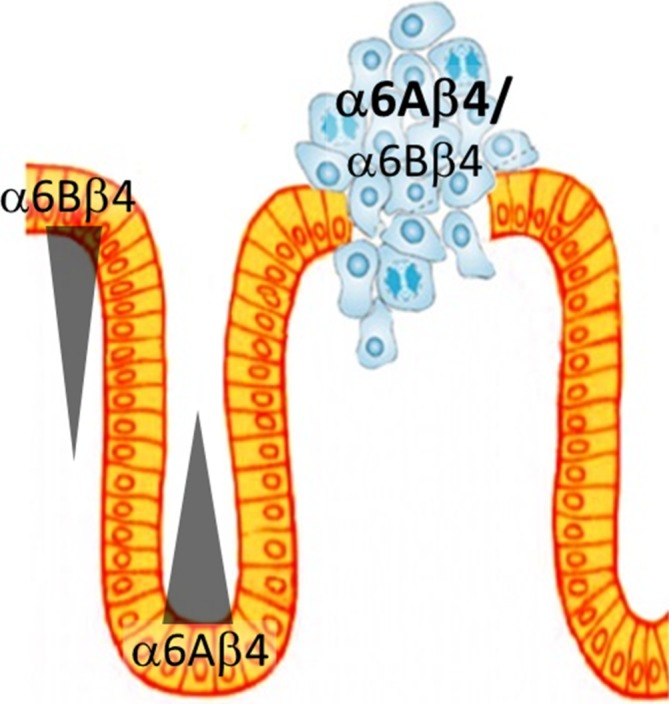
Schematic view of the colonic epithelium showing the differential expression of integrin α6β4 forms in the normal mucosa and in tumours. Under normal conditions, the α6A subunit is located in the proliferative cells of the gland while α6B is confined to the quiescent upper gland and surface epithelial cells.5 In colorectal tumours, total α6β4 expression increases as its α6Aβ4 form.5 8
In relation to these previously reported observations, one should also consider the likelihood that an abolition/loss of the α6β4 heterodimer in the gut epithelium favours compensatory cell–matrix interactions through other receptors such as the pro-proliferative 37/67-laminin receptor9 and other previously identified pro-proliferative integrins,10 which, singly or in combination, may contribute to the complex phenotype that has been observed in the experimental mouse mutant models described.1
In this context, the colon tumorigenesis observed in mice carrying a total gut epithelial-specific deletion of the α6 integrin subunits may need to be interpreted with caution.
Acknowledgments
The author thanks Professor Pierre Vachon for the critical reading of the manuscript and suggestions and Elizabeth Herring for corrections.
Footnotes
Funding: The original work was funded by the Canadian Institutes of Health Research grants MOP-97836 and MOP-123415 and the Canada Research Chair in Intestinal Physiopathology.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. De Arcangelis A, Hamade H, Alpy F, et al. . Hemidesmosome integrity protects the colon against colitis and colorectal cancer. Gut 2017;66:1748–60. 10.1136/gutjnl-2015-310847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramovs V, Te Molder L, Sonnenberg A. The opposing roles of laminin-binding integrins in cancer. Matrix Biol 2017;57-58:213–43. 10.1016/j.matbio.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 3. Stewart RL, O’Connor KL. Clinical significance of the integrin α6β4 in human malignancies. Lab Invest 2015;95:976–86. 10.1038/labinvest.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goel HL, Gritsko T, Pursell B, et al. . Regulated splicing of the α6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Rep 2014;7:747–61. 10.1016/j.celrep.2014.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dydensborg AB, Teller IC, Groulx JF, et al. . Integrin alpha6Bbeta4 inhibits colon cancer cell proliferation and c-Myc activity. BMC Cancer 2009;9:223 10.1186/1471-2407-9-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dydensborg AB, Teller IC, Basora N, et al. . Differential expression of the integrins alpha6Abeta4 and alpha6Bbeta4 along the crypt-villus axis in the human small intestine. Histochem Cell Biol 2009;131:531–6. 10.1007/s00418-008-0547-z [DOI] [PubMed] [Google Scholar]
- 7. Ni H, Dydensborg AB, Herring FE, et al. . Upregulation of a functional form of the beta4 integrin subunit in colorectal cancers correlates with c-Myc expression. Oncogene 2005;24:6820–9. 10.1038/sj.onc.1208848 [DOI] [PubMed] [Google Scholar]
- 8. Groulx JF, Giroux V, Beauséjour M, et al. . Integrin α6A splice variant regulates proliferation and the Wnt/β-catenin pathway in human colorectal cancer cells. Carcinogenesis 2014;35:1217–27. 10.1093/carcin/bgu006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khalfaoui T, Groulx JF, Sabra G, et al. . Laminin receptor 37/67LR regulates adhesion and proliferation of normal human intestinal epithelial cells. PLoS One 2013;8:e74337 10.1371/journal.pone.0074337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boudjadi S, Bernatchez G, Sénicourt B, et al. . Involvement of the Integrin α1β1 in the progression of colorectal cancer. Cancers 2017;9:96 10.3390/cancers9080096 [DOI] [PMC free article] [PubMed] [Google Scholar]


