Abstract
Background
The bursting pattern of thalamocortical (TC) pathway dampens nociception. Whether brain stimulation mimicking endogenous patterns can engage similar sensory gating processes in the cortex and reduce nociceptive behaviors remains uninvestigated.
Objective
We investigated the role of cortical parvalbumin expressing (PV) interneurons within the TC circuit in gating nociception and their selective response to TC burst patterns. We then tested if transcranial magnetic stimulation (TMS) patterned on endogenous nociceptive TC bursting modulate nociceptive behaviors.
Methods
The switching of TC neurons between tonic (single spike) and burst (high frequency spikes) firing modes may be a critical component in modulating nociceptive signals. Deep brain electrical stimulation of TC neurons and immunohistochemistry were used to examine the differential influence of each firing mode on cortical PV interneuron activity. Optogenetic stimulation of cortical PV interneurons assessed a direct role in nociceptive modulation. A new TMS protocol mimicking thalamic burst firing patterns, contrasted with conventional continuous and intermittent theta burst protocols, tested if TMS patterned on endogenous TC activity reduces nociceptive behaviors in mice.
Results
Immunohistochemical evidence confirmed that burst, but not tonic, deep brain stimulation of TC neurons increased the activity of PV interneurons in the cortex. Both optogenetic activation of PV interneurons and TMS protocol mimicking thalamic burst reduced nociceptive behaviors.
Conclusions
Our findings suggest that burst firing of TC neurons recruits PV interneurons in the cortex to reduce nociceptive behaviors and that neuromodulation mimicking thalamic burst firing may be useful for modulating nociception.
Keywords: electrical therapy, rTMS, thalamic bursting, nociception, parvalbumin interneurons, sensory gating, bioelectric medicine
Introduction
The investigation of electrical brain stimulation to control central pain is long-standing and often empirical [1–3]. Generally, the mechanism of pain relief is based on interfering with neuronal circuits responsible for pain processing or perception [4, 5] with synthetic brain stimulation patterns intended to override endogenous activity. Could novel brain stimulation strategies that correct circuit pathology with patterns emulating endogenous activity enhance therapeutic efficacy? Such an approach derives from a precise hypothesis on disease etiology to identify 1) firing patterns correlated with suppression of pain and, 2) the cellular targets of that patterned activity that mediate pain processing.
Nociception serves vital protective functions against bodily injury. As part of the TC circuit, the sensory thalamus plays a critical role in gating transmission of peripheral nociceptive information to the somatosensory cortex, where representation and perception of pain is assumed to occur [6–10]. This sensory gating function of the thalamus has been suggested to be mediated by the ability of individual TC neurons to fire in tonic and burst firing modes via interconnections with the cortex and thalamic reticular nucleus (TRN) [11–14]. Specifically, the γ-aminobutyric acid (GABAergic) projection from TRN to TC neurons de-inactivates T-type calcium channels, inducing strong inhibition that, in turn, leads to low threshold calcium spike “rebound” bursts [15]. Subsequent in-vivo studies suggests that the tonic firing of TC neurons correlates with nociceptive responses [16–18] while the burst firing of TC neurons correlates with suppression of pain responses [16, 19–21].
Although studies suggested differential roles for TC tonic and burst firing in pain processing, how the dual firing modes of TC neurons contribute to differential pain processing in the somatosensory cortex, which should be a crucial part of an ascending pain control mechanism, is currently unknown. The sensory cortex is a highly organized structure with layer specific input/outputs and the sensory TC neurons, which directly receive peripheral sensory inputs, primarily synapse onto layer 4 of the cortex [22]. Of the two firing modes of TC neurons, burst firing, compared to tonic firing, has been shown to have greater potency to activate inhibitory interneurons in the cortex [23, 24].
Among the interneuron types expressed in the cortex, PV expressing inhibitory interneurons are especially suited to exert feed-forward inhibition to excitatory pyramidal neurons. Of the two main type of GABAergic interneurons expressed in layer 4 of the cortex, PV interneurons are more abundant (constituting 60% of GABAergic interneurons) than somatostatin (SOM) expressing interneurons (constituting 20–30% of GABAergic interneurons) [25, 26]. PV interneurons are fast-spiking and synapse onto proximal dendrites or somatic regions of pyramidal neurons [27, 28]. Cortical PV interneurons are directly innervated by thalamic projections [29, 30] while SOM interneurons only have weak connections with thalamic inputs [31, 32]. Together, these properties make PV interneurons ideal for implementing feed-forward inhibition [29] that can be driven by high frequency TC burst firing.
Activity of PV interneurons is reduced or disrupted in the somatosensory cortex of mice with neuropathic pain [33, 34] and SOM activation can alleviate neuropathic pain associated allodynia [34]. However, the role of cortical PV interneurons within the TC circuit in gating nociception of non-neuropathic conditions remains uninvestigated. In particular, a circuit level mechanism of how the dynamics between TC tonic and burst dual firings modulate nociception at the cortical level is unknown. The present study examined whether burst, but not tonic, firing mode of TC neurons engages cortical PV interneurons to exert inhibitory modulation of pyramidal neurons in the primary (S1) somatosensory cortex and whether activation of cortical PV interneurons could behaviorally suppress nociceptive responses in mice.
Using electrical stimulation and immunohistochemical methods we investigated whether burst stimulation of TC neurons could significantly activate PV interneurons in the sensory cortex compared to tonic stimulation or sham control conditions. Next we tested whether selective activation or inactivation of cortical PV interneurons with optogenetic or patterned transcranial magnetic stimulations could modulate nociceptive behaviors in mice.
Materials and methods
Animals
Optogenetic experiments employed PV-Cre male mice (8–16 weeks; Jackson Laboratories). All other studies used first generation 129/SvJae × C57BL/6J hybrid mice (male, 8–12 weeks). Mice were group-housed and maintained at 12 h light-dark cycle (lights on at 8 AM) with free access to food and water. Following a surgery, animals were singly-housed. All experiments were conducted in compliance with the Animal Care and Use Committee (Approval number: AP 2015025). Mice were randomly assigned to experimental groups and based on histology animals with misplaced electrodes or viral injections were excluded from analyses.
Surgical procedures
All surgical procedures were performed under anesthesia (30 mg/kg Zoletil, IP) and using a stereotaxic instrument (Kopf Instruments) with brain coordinates based on the Paxinos and Franklin (2001) mouse brain atlas [35]. Animals were given Ketoprofen (5 mg/kg SC) right after surgery and daily for a week for post-operative recovery.
For electrical stimulation of the ventroposterolateral (VPL) thalamus, two bipolar stimulating electrodes (0.6 mm apart; Teflon-coated stainless steel, 0.003” bare 0.055” coated, A-M Systems) were implanted (AP: −1.34 mm, ML: −1.85 mm, DV: −3.2 mm). The electrodes were secured onto the skull with stainless steel screws and dental cement.
For optogenetic experiments, AAV-DIO-ChR2–eYFP and AAV-DIO–eYFP purchased from the University of North Carolina Vector Core were injected into the primary somatosensory cortex corresponding to the hind limb region (S1HL; AP: −0.5 mm, ML: −1.64 mm, DV: −0.5 mm). The virus injections were made slightly lateral to the optic fiber implantation site, avoiding major arteries. Using glass pipettes (tip size 20–38 µm), a total of 200 nl was injected over 10 min using a Nanoliter Injector (World Precision Instruments). After a week of recovery, an optic fiber (GIF 625; Thor Labs) was chronically implanted into the S1HL (AP: −0.5 mm, ML: −1.6 mm, DV: −0.4 mm).
For TMS, a plastic baseplate was permanently affixed to the skull with Loctite 454 and dental cement. Later, a solenoid coil was connected to the baseplate for magnetic stimulation centering on the S1HL (AP: −0.5 mm, ML: −1.6 mm).
Electrical stimulation
Mice were habituated to tethering, mockup IP injection (using a syringe without needle), and the experimental apparatus for 30 min daily for a week. On the experiment day, mice were anesthetized with urethane (1.5 g/kg IP), connected to a stimulation cable, and after 10 min received either tonic or burst stimulation for 5 min. Mice in the sham control group were attached to the stimulation cable for the same duration without receiving stimulations. All stimulating pulses were biphasic square pulses with 100 µA current amplitude and 100 µs duration. Burst stimulation consisted of 3 ms intervals of 5 burst pulses with 600 ms interval between the 5 burst pulses, while tonic stimulation was 600 pulses at 2 Hz.
Immunohistochemistry
Mice were anesthetized with urethane (1.5 g/kg IP) and brains were extracted after transcardial perfusion with physiological saline (0.9%) followed by 10% formalin solution diluted in physiological saline at room temperature. Brains were then successively placed in 10% formalin solution for a day at 4 °C and 30% sucrose solution for two days at 4 °C, before being cut in coronal sections (40 µm) with a cryostat (Microm). Free floating sections were processed for standard immunohistochemical procedures, mounted on microscope slides, and images were acquired with a fluorescent microscope (Zeiss AxioImager M2) and a confocal microscope (Olympus FluoView FV 1000) for analysis.
For the electrical stimulation experiment, mice were perfused 90 min after stimulation and processed for cFos and parvalbumin double fluorescence labeling. Free floating brain sections were blocked with 10% normal donkey serum (NDS) in PBS containing 0.3% Triton X-100 for 2 h at room temperature and incubated in a mixture of 1:1000 rabbit anti-parvalbumin (Abcam; ab11427) and 1:100 goat anti-cFos (Santa Cruz Biotechnology; sc-52-G) in 3% NDS diluted with PBS for 72 h at 4 °C. The brain sections were then incubated for 1 h with 1:200 AlexaFluor 568 anti-rabbit (Invitrogen; A10042), and 1:200 Alexafluor 488 anti-goat (Invitrogen; A11055).
For verification of expression location and specificity of PV neurons expressing ChR2, PV-Cre mice injected with AAV-DIO-ChR2–eYFP virus were double labelled for PV and eYFP. Coronal brain sections (40 µm) cut through the S1HL region were blocked with 10% NDS in PBS containing 0.1% Triton X-100 for 2h at room temperature and incubated in a mixture of 1:1000 rabbit anti-parvalbumin (Abcam; ab11427) and 1:1000 chicken anti-YFP (Abcam; ab13970) overnight at room temperature. The brain sections were then incubated for 2 h with 1:200 Alexa 488 anti-chicken (Jackson ImmunoResearch; 103-545-155) and 1:200 Alexa 594 anti-rabbit (Vector Laboratories; DI-1594).
Analysis of immunolabeled neurons
Images (317 × 317 µm size) of the S1HL region from AP −0.46 mm to 0.82 mm in layer 4 and 5 were obtained with a confocal microscope using FluoView FV 1000 (Olympus). Laser settings were kept constant throughout the whole experiment. The number of cFos and PV labeled cells were manually counted by two investigators blind to groups using the Olympus FV10-ASW ver.4.1a Viewer.
Behavioral nociception tests
Electronic von Frey, plantar and formalin tests were used to gauge acute and tonic nociception changes to optical and magnetic stimulations. All brain stimulations were given in the right hemisphere. Before behavioral tests, mice were handled and habituated to the experimental background for 30 min/day for a week. All experiments were performed under blind conditions to the background of mice (ChR2 or eYFP expression) and the type of magnetic stimulation delivered (intermittent theta-burst-stimulation: iTBS, continuous theta-burst-stimlation: cTBS, or ‘Thalamic burst’).
Electronic von Frey (IITC Inc.) was used to measure hind paw withdrawal thresholds. Two measurements were made each for baseline and during stimulations. Measurements were taken at 20 min interval for each paw. Optical stimulation was delivered at 20 Hz (tip power 0.8 mW) with a 473 nm blue laser diode (Shanghai Dream Lasers) just before measuring threshold changes induced by light stimulation and immediately turned off after making a measurement. For the magnetic stimulation, TMS was turned on for 600 pulses before the first measurement and stayed on until a set number of pulses were delivered.
Thermal thresholds were measured with a plantar test apparatus (Ugo Basile). Infrared (IR) intensity was set at 60 and cut off time was 33 s. Hind paw withdrawal latency was measured at 30 min intervals. Two measurements were taken for baseline and during stimulations. Same optical and magnetic stimulation methods as the von Frey experiment was used.
Formalin-induced nociception was induced by injecting 10 µl of 5% formalin solution diluted in saline (0.9%) to the left hind paw with a syringe attached to a customized 28 G needle (Hamilton). Optical stimulation (1ms at 20 Hz, 473 nm light, 0.8 mW at tip) was turned on for 0–5 min and 15–30 min segments during the formalin test, which corresponds to the two peaks of nociceptive behaviors. Behaviors were videotaped and the duration of licking and shaking were scored in 5 min blocks by two investigators blind to groups.
TMS coil design and rTMS protocol
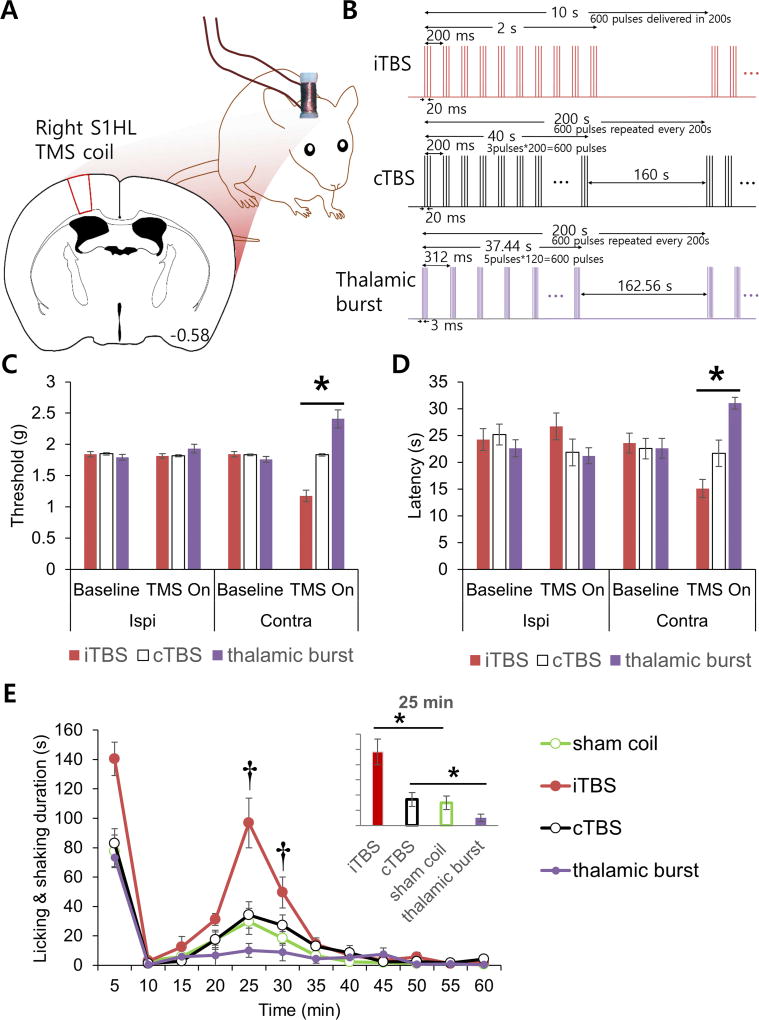
A solenoid coil (4 mm diameter × 10 mm height) made of 113 wounding of 0.3 mm enamel insulated copper wire was used for TMS. A plastic support stand was designed to anchor a solenoid coil to a baseplate. The weight of a baseplate, a support stand, and a coil was approximately 2 g. High frequency alternating current (125 kHz, 800 mA) was used for repetitive TMS (rTMS). TMS protocols used were, iTBS and cTBS, adapted from Haung et al. (2006) [36], and ‘Thalamic burst’, a new stimulation protocol devised based on thalamic burst firing patterns. For iTBS, 3 pulses at 50 Hz were repeated every 200 ms for 2 s with 8 s pause between bursts (Fig. 3b). For cTBS, 3 pulses at 50 Hz were repeated every 200 ms for 40 s with a 160 s pause between stimulations. For ‘Thalamic burst’, 5 pulses at 333 Hz (3 ms between pulses) were repeated every 314 ms for 37.44 s with a 162.56 s pause between stimulations. Equal number of pulses were delivered for iTBS, cTBS, and ‘Thalamic burst’: a total of 4200 pulses were delivered during the 25 min stimulation time in von Frey tests and a total of 6000 pulses were delivered during the 35 min stimulation time in plantar and formalin tests. Pulse duration was 10 ms for iTBS and CTBS, while pulse duration for ‘Thalamic burst’ was 2 ms, due to a short interval between stimulation pulses.
Fig. 3.
TMS of the cortex and nociception. (A) Schematic drawing of TMS coil placement. TMS was given in awake behaving mice during nociception tests. (B) The total numbers of iTBS, cTBS, and thalamic burst pulses were matched for each behavioral test (600 pulses/cycle, 1 cycle = 3 min 20 s). Figure not drawn to scale. (C) Mechanical threshold changes induced by different TMS protocols. Paw withdrawal thresholds were measure with von Frey filaments (Kruskal-Wallis test with Mann-Whitney U test; n = 6 mice per group). (D) Acute thermal nociception changes triggered by TMS protocols. Paw withdrawal latency to plantar paw IR irradiation (Kruskal-Wallis test with Mann-Whitney U test; n = 6 mice per group). (E) Formalin-induced inflammatory nociceptive behavior changes to different TMS protocols (repeated measures ANOVA followed by Games Howell post hoc; n = 6 mice per group). All data are shown as mean ± SEM. *P < 0.05 between groups indicated by horizontal lines. †P < 0.05 between the ‘thalamic burst’ and the ‘sham coil’ groups.
In vitro electrophysiology
Under isoflurane anesthesia, the mouse brains were rapidly extracted, and coronal sections (300 µm) were made using a vibratome (Leica) in ice cold ACSF (in mM: NaCl2 130, KCl 3.5, MgCl2 1, CaCl2 1.5, NaH2PO4 1.25, NaHCO3 25, glucose 10). Slices were then incubated in ACSF saturated with 95% O2 and 5% CO2 for at least an hour before commencing whole cell recordings using a MultiClamp700B (Molecular Devices). The internal solution of glass recording electrodes contained 130 K-gluconate, 15 KCl, 5 NaCl, 5 Mg-ATP, 1 MgCl2, 5 EGTA, 1 CaCl2, and 10 HEPES, pH 7.2 (300 mOsm). All recordings were done at room temperature.
Data Analysis
Statistical significances were assessed using repeated measures ANOVA followed by Games Howell post hoc test when comparing changes over time, and one-way ANOVA with Tukey HSD when comparing more than two means, except where noted. For data with normal distribution and unequal variance, Welch’s ANOVA and Games-Howell post hoc test were performed. In cases where Levene’s Test of Homogeneity of Variance was significant, Kruskal-Wallis and Mann-Whitney tests were used to test for group differences. Significance was determined at *p<0.05. Appropriate group sizes were determined by a power analysis (G*Power 3.1), using an alpha of 0.05, power of 0.8, and effect size (Cohen’s f) of 0.4 for F tests and 0.8 for t tests [37]. Analyses were performed with SPSS 13.0 and graphs were plotted with Microsoft Excel.
Results
Thalamic firing modes differentially affect the activity of cortical PV interneurons
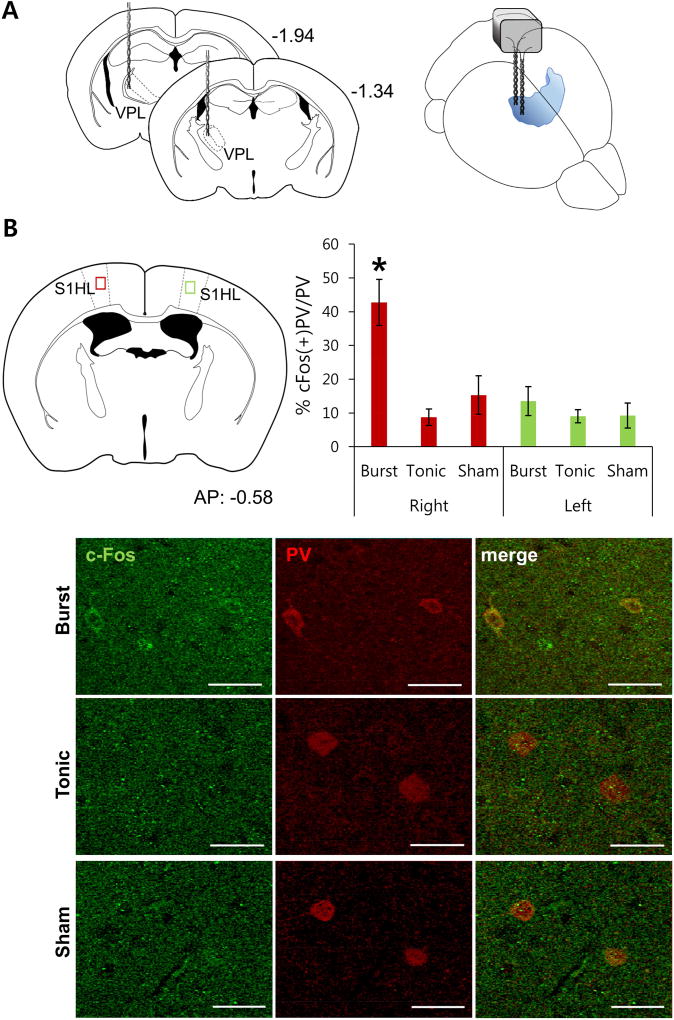
To determine the relationship between thalamic firing patterns and cortical PV interneuron activity, two bipolar stimulating electrodes were implanted into each animal’s right ventroposterolateral (VPL) thalamus (Fig. 1A). Mice were assigned to either burst (5 pulses at 333 Hz with 600 ms inter-burst interval; known to produce antinociceptive effects [16, 19]), tonic (2 Hz), which does not induce any antinociceptive effect (data not shown), or sham control (no stimulation) groups. Stimulation procedures were carried out under urethane anesthesia to minimize the influence of sensory signals from confounding the results. The number of cFos and PV expressing neurons were quantified in layers 4/5a of the primary somatosensory cortex, corresponding to the hind limb (S1HL), which receives extensive inputs from the ipsilateral primary sensory thalamus [11, 22]. While the number of cFos or PV expressing neurons in the right S1HL was similar between the three groups (Supplementary Fig. 1), the number of neurons co-expressing cFos and PV was significantly elevated only in animals that received burst stimulation. Specifically, >40% of PV interneurons in the right S1HL expressed cFos in response to burst stimulation of the right VPL, compared to 9% and 15% of PV interneurons co-expressing cFos in tonic stimulation and sham control groups, respectively (Fig. 1B). Further, the increased cFos/PV co-expression observed in the burst stimulation groups was confined to the ipsilateral hemisphere, which corresponded to antinociceptive effects on the contralateral body regions (see below Fig. 2C). The fact that the total number of cFos expressing neurons in layers 4/5a of the primary somatosensory cortex did not differ following different stimulation patterns to the VPL suggests that more non-PV neurons expressed cFos in tonic stimulation and sham control groups. Presumably, in the burst stimulation group, the increased PV interneuron activity (cFos/PV co-expression) reflects suppression of non-PV neuron activity (cFos only expression). These immunohistochemical results indicate that burst firing activities of the thalamus, which inhibit nociceptive responses, preferentially activate cortical PV interneurons.
Fig. 1.
Activation of cortical PV interneurons to electrical stimulation of the thalamus. (A) Schematic drawings of stimulating electrodes placed in the right VPL. (B) The red and green squares show the locations of the somatosensory cortex examined for cFos and PV double staining after electrical stimulation of the thalamus (top left). They also represent ipsi- and contralateral hemispheres to the stimulation site, respectively. Bar graph shows the percentage of cFos positive PV neurons to different types of electrical stimulations (top right). Bars indicate mean ± SEM * P < 0.05, Welch’s ANOVA with Games-Howell post hoc indicated significant difference between burst and other groups. Bottom images shows representative samples of cFos and PV staining for 3 groups of electrical stimulations. (n = 3 mice per group, 3–4 slices per mouse)
Fig. 2.
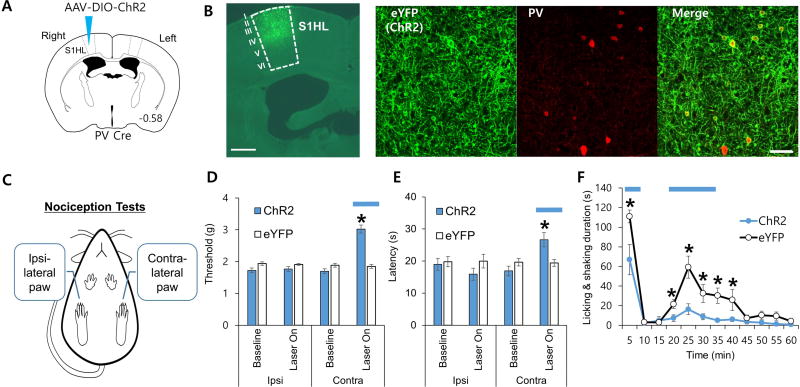
The effects of optogenetic manipulation of PV interneurons in the primary sensory cortex on various nociception tests. (A) Schematic drawing of AAV-DIO-ChR2 injected into the right hemisphere of PV Cre mice. (B) Images showing ChR2 expressions in the upper layers of the S1HL region (left-most panel, 500 µm scale; three panels, 50 µm scale). (C) Nociceptive threshold changes to PV interneuron activation were measured for both paws in acute pain tests. Behavioral nociception was measured only for the left paw during the longer lasting formalin induced nociception. (D–F) Blue horizontal lines indicate blue light stimulation in the right hemisphere which affected the left paw, since nociceptive signals are transmitted contralaterally.
(D) Mechanical threshold changes triggered by blue light stimulation were measured via von Frey (Welch’s ANOVA with Games-Howell post hoc; ChR2, n = 6 mice; eYFP, n = 4 mice). (E) Acute thermal nociception changes triggered by blue light stimulation were measured via plantar test (Kruskal-Wallis test with Mann-Whitney U test; ChR2, n = 6 mice; eYFP, n = 4 mice). (F) Response to inflammatory nociception induced by formalin injection in the left paw. Blue light stimulation was given during the 0–5 min and 15–30 min segments as indicated with blue horizontal bars (repeated measures ANOVA followed by Games Howell post hoc; ChR2, n = 8 mice; eYFP, n = 8 mice). Data are presented as mean ± SEM, *P < 0.05
Optogenetic activation of cortical PV interneurons decreases nociceptive behaviors
Next, we examined whether bypassing the VPL and directly activating PV interneurons can modulate nociceptive responses. To do so, double floxed (DIO) Cre-dependent adeno-associated virus (AAV) expressing channelrhodopsin-2 (AAV-DIO-ChR2-eYFP) or control virus (AAV-DIO-eYFP) was injected into the right S1HL of PV-Cre transgenic mice (Fig. 2A). ChR2 was expressed robustly and selectively in PV interneurons (90±3%) in layers 1–5 of the S1HL region within AP 0.38 mm to AP −1.34 mm range (Fig. 2B and Supplementary Fig. 2). Following confirmation of functional expression of ChR2 via in vitro whole cell electrophysiology (Supplementary Fig. 3), PV interneurons in the right S1HL were optogenetically stimulated while performing several pain tests including the von Frey (acute mechanical), plantar (acute thermal) and formalin (lasting inflammatory) tests, to demonstrate that PV interneurons in the cortex are critically involved in modulating pain thresholds. Specifically, nociceptive thresholds of ipsilateral and contralateral paws were compared during optical stimulation to verify contralateralization of peripheral nociceptive information to the VPL-S1HL circuit (Fig. 2C). A light stimulation protocol of 1 ms pulses of blue light (473 nm) delivered at 20 Hz significantly elevated mechanical and thermal nociceptive thresholds in the contralateral paw compared to the ipsilateral control paw (Fig. 2D and 2E). Inflammatory nociceptive responses induced by formalin injection in the contralateral paw were also significantly reduced by activation of cortical PV interneurons (Fig. 2F). Although ChR2 was expressed across layers 1–5 in S1HL, because the optic fiber tip was in layer 4 and because the estimated light density drops from 257 mW/mm2 at the tip to 29 mW/mm2 at 150 µm distance [38, 39], the present antinociceptive effects are largely due to stimulation of PV interneurons in the layer 4. The fact that selective activation of PV interneurons can suppress diverse pain behaviors, strongly support the hypothesis that PV interneurons in the S1 are essential in gating nociception.
TMS protocol mimicking thalamic bursts reduces nociceptive behaviors
Since thalamic burst firings reduced nociceptive behavior by affecting the activity of cortical inhibitory interneurons, we tested whether TMS protocol mimicking thalamic bursts, ‘Thalamic burst’, could also reduce nociceptive behaviors. To test this, the ability of a newly developed ‘Thalamic burst’ protocol to modulate nociceptive behaviors were compared with those of established protocols, iTBS and cTBS. A customized miniature TMS coil (Fig. 3A) was secured above the right hemisphere to apply magnetic stimulation. Nociceptive thresholds were measured before and after delivering identical number of pulses to keep the total number of stimulating pulses equal among stimulation protocols (see methods for details). ‘Thalamic burst’, iTBS, or cTBS was applied to separate groups of animals to assess whether non-invasive method could actually modulate nociceptive behaviors. ‘Thalamic burst’ significantly decreased nociceptive responses of the contralateral paw in von Frey (mechanical) and plantar (thermal) tests (Fig. 3C and 3D). Formalin induced nociceptive behaviors were also significantly reduced by ‘Thalamic burst’ (Fig. 3E). The first phase behavioral responses (0–5 min) did not differ, but the second phase responses (20–25 min) significantly differed from the other groups (Fig. 3E, bar graph). In contrast, iTBS, which was reported to decrease the activity of cortical PV interneurons [40, 41], significantly enhanced nociceptive behaviors in both the first (0–5 min) and second phases (25–30 min), while cTBS has no significant effect (Fig. 3C–E). These rTMS results support that nociceptive behaviors could be differentially modulated by TMS protocols designed to mimic endogenous thalamocortical input.
Discussion
Circuit based neuromodulation
The treatment of pain disorders is among the most long-standing, technologically diverse, and prevalent applications for neuromodulation [42–44]. Brain stimulation anatomical targets have been justified by contemporaneous theories of pain for example gate-control by activation of peripheral and central afferents, stimulated release of endogenous opioids by stimulation of deep nuclei, modulation of sensory integration by thalamic stimulation, modulation of sensory perception by motor cortex stimulation, or modulation of pain by frontal cortex stimulation [3, 45, 46]. Technological advancement has similarly focused on new anatomical targets (e.g. invasive and non-invasive forms of current delivery) including new implanted leads [47, 48], magnetic induction [49, 50], and transcranial electrical targeting [51]. The exploration of waveforms has been relatively limited, often exploring variations in the frequency of tonic stimulation or adopting canonical patterns demonstrated to produce plasticity in human and animal neurophysiology (e.g. theta burst, direct current). Approaches using more customized waveforms are investigated [52, 53] including closed-loop approaches [54–58]
A circuit based approach to neuromodulation [59, 60] involves consideration of both the anatomical target and waveform, in the context of pathological network activity. The approach taken here was to characterize a network associated with dampening of nociceptive responses and to design brain stimulation strategies to engage this same network activity.
TC circuit mechanism of nociceptive gating
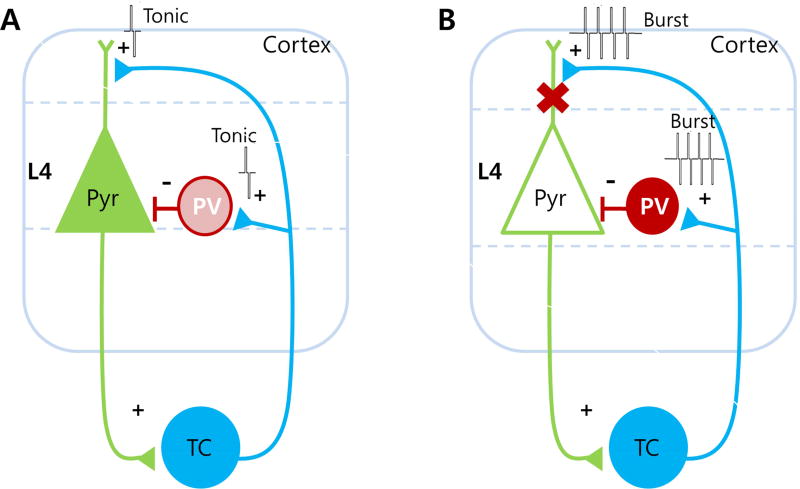
We believe the present study provides direct evidence of a novel TC circuit mechanism of nociceptive signal gating that involves PV interneurons in the somatosensory cortex (Fig. 4). To implement the thalamic sensory gating role, tonic and burst firing of TC neurons are likely to activate different cellular substrates in the cortex. Specifically, our model predicts that tonic firing of TC neurons (conveying pain information) will produce excitatory post-synaptic responses on pyramidal neurons in the sensory cortex while insignificantly affecting the activity of PV interneurons (Fig. 4A). In contrast, high frequency burst firing of TC neurons is predicted to preferentially activate cortical PV interneurons to decrease nociception (Fig. 4B).
Fig. 4.
A putative role of cortical PV interneurons in modulating nociceptive signals within the thalamocortical circuit. (A) Nociceptive signal transmission during TC neuronal tonic firing. (B) Inhibition of nociceptive signal transmission to cortical pyramidal (Pyr) neurons during TC neuronal burst firing. The filled Pyr neuron represents activation while empty Pyr neuron represents inactivation. The gradient of the PV color indicates the level of activation with darker color indicating greater activation.
The exact mechanisms of how TC tonic firing could preferentially activate pyramidal neurons over inhibitory interneurons are unclear, but nociceptive signals may trigger TC neurons to fire in a synchronized tonic mode to excite excitatory neurons in cortical layer 4. Indeed, a previous study showed that synchronously active tonic firings of TC neurons were able to excite excitatory neurons in the cortical layer 4 [61]. Conversely, high frequency stimulation of TC neurons was reported to depress TC synapses [62]. Although direct evidence linking TC tonic firing and cortical pyramidal activity is missing, independent studies showed that increasing TC tonic firing enhanced pain [17, 18] and that allodynia in neuropathic pain models was associated with increased activity of pyramidal neurons in the somatosensory cortex [34]. These studies suggest that increased TC tonic activity and cortical excitation could both contribute to enhanced pain behavior.
Burst firing of TC neurons, on the other hand, is likely to preferentially activate cortical PV interneurons to decrease nociception (Fig. 4B). Our data showed that burst stimulation of TC neurons significantly increased activation of PV interneurons in the somatosensory cortex and selective activation of them significantly reduced nociceptive behaviors. Although the role of TC burst firing in pathological pain is still controversial [6], recent papers suggest that burst firing of TC neurons acts to diminish nociceptive pain in non-pathological conditions [16, 19–21], and our data provides evidence on how thalamic burst firing could attenuate nociception at the cortical level by a specific type of inhibitory interneuron: PV interneurons.
Among the various types of interneurons expressed in the cortex, PV interneurons are most suitable to exert powerful inhibition onto cortical pyramidal neurons to reduce nociceptive signals. Not only are they the most abundantly expressed inhibitory interneuron in layer 4 of the somatosensory cortex [25, 63] which directly receives sensory information, but also their fast-spiking properties make them ideal for feed-forward and feed-back inhibition [29] to attenuate excessive excitatory activity. They are also interconnected via gap junctions, enabling these neurons to exert a synchronized inhibition onto pyramidal neurons in the sensory cortex [64]. Enhanced excitation in the somatosensory cortex was reported in chronic pain models [34, 65, 66], suggesting that reduced inhibition on pyramidal neurons could lead to pathological pain symptoms. More specifically, PV interneuron connectivity was found to be disrupted in the primary somatosensory cortex of multiple sclerosis chronic neuroinflammatory model [33]. Peri-neuronal nets (PNNs), a specialized extracellular matrix that enables PV interneurons to fire in high frequencies [67, 68], were also found to be significantly reduced in the same study [33], emphasizing the importance of functionally active PV interneurons in controlling pain.
Cell-type specificity
Although increased excitatory activity was reported to be associated with increased nociceptive behavior, enhanced GABAergic activity itself was insufficient to overcome neuropathic pain symptoms, since the activities of both excitatory and inhibitory cortical neurons were enhanced in a chronic pain model [66]. This may in part be due to the difference in activation pattern of different types of inhibitory interneurons in the cortex, which have different roles. In support of this prediction, a recent study showed that the activities of cortical inhibitory interneurons that express SOM and PV were reduced while those expressing vasoactive intestinal polypeptide (VIP) was enhanced [34]. When SOM neurons were selectively activated, mechanical allodynia induced by neuropathic pain was reduced, supporting the importance of targeting a specific type of neurons for treating pain.
Understanding the cellular targets of neuromodulation (which cellular elements are stimulated; [69]) is pivotal to mechanism-based interventions [70, 71]. Long-standing efforts to optimize targeting of specific cell types is intended to enhance specificity in outcomes [72, 73]; while peripheral stimulation focuses on selecting axon types [74, 75], selectivity in the CNS is complicated by the diversity of morphology and interconnectivity of neurons [76]. iTBS was suggested to reduce the activity of cortical PV interneurons while cTBS reduces the activity of cortical SOM interneurons [40, 41]. The present finding that iTBS to the somatosensory exacerbated nociceptive behaviors in mice further supports the role of cortical PV interneurons in gating nociceptive signals.
We developed a new stimulation protocol based on thalamic burst firing patterns and targets, ‘Thalamic burst’ TMS, which was shown to have an antinociceptive effect in mice. This is consistent with the approach that stimulation protocols developed based on brain activity patterns may be useful for modulating specific behaviors. Considering that different stimulation patterns leads to activation of different cell types in the brain [41, 77, 78], the new stimulation protocol putatively activated cortical PV interneurons, but this remains to be verified.
Modulatory effect of brain stimulations
Several neuromodulation methods—deep brain stimulation (DBS), motor cortex stimulation (MCS), transcranial direct current stimulation (tDCS), or TMS—have been shown to be effective in modulating pain [4, 79, 80]. Exact mechanisms of action of these stimulation methods are not completely understood but may active endogenous (e.g. opioid) regulatory systems [81, 82]. Any of these techniques to deliver electricity to the brain may benefit from incorporating endogenous patterns, provided it is symptom etiology and target circuit specific. When stimulation leads to lasting changes (e.g. clinical benefit after a stimulation session) this approach should be linked to identifying underlying neuroplasticity or molecular changes. For example, modulatory effect of brain stimulations may occur partly by influencing glia [83–88], since glial cells in the brain, especially astrocytes and microglia, are closely related to chronic pain [89, 90].
Limitations
The limitations of the present work include those universal to any animal model of diseases. Nonetheless, mechanisms and interventions found relevant in nociception of mice, including the three behaviors tested here, have provided useful translational predictions [91]. There are further inherent limitations in translating the brain stimulation protocols here to clinical use, namely TMS is less focal in rodent models [92–94] even as we developed a specialized coil. However, the differentiations we show in regard to both waveform pattern and laterality, buttress overall conclusions on specificity and targeting. Finally, it is important to recognize that precisely because we suggest matching neuromodulation strategy to endogenous pain networks, diverse pain etiology would suggest distinct interventional strategies.
Conclusion
Overall, our findings show that brain stimulations strategies mimicking endogenous TC activity dampen nociceptive behaviors in mice, supporting further investigation of targeted circuit-based neuromodulation interventions for pain.
Supplementary Material
Highlights.
Electrical stimulation mimicking thalamic bursts activate cortical PV interneurons
Selective optical activation of PV interneurons reduces nociceptive behaviors
TMS mimicking ‘Thalamic burst’ reduces nociceptive behaviors in mice
Acknowledgments
We thank Nakajima Ryuichi and Bradley Baker for providing PV-Cre mice, and Frances Cho and Michael M. Morgan for helpful discussion and comments on the manuscript.
Funding
This research was supported by the Ministry of Science and ICT through the National Research Foundation of Korea (NRF) grants: Mid-career Researcher Program (NRF-2015R1A2A2A04005487) and Brain Science Research Program (NRF-2015M3C7A1028392). This study was also funded by NIH grant MH099073.
Abbreviations
- cTBS
continuous theta-burst-stimulation
- iTBS
intermittent theta-burst-stimulation
- PV
parvalbumin expressing
- PNNs
Peri-neuronal nets
- rTMS
repetitive transcranial magnetic stimulation
- S1
primary somatosensory cortex
- SOM
somatostatin
- TC
thalamocortical
- TMS
transcranial magnetic stimulation
- TRN
thalamic reticular nucleus
- VPL
ventroposterolateral
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
Authors confirm that there were no known conflicts of interest associated with this work and there were no financial support for this work that could have influenced its outcome.
References
- 1.Cruccu G, Aziz TZ, Garcia-Larrea L, Hansson P, Jensen TS, Lefaucheur JP, et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007 Sep;14(9):952–70. doi: 10.1111/j.1468-1331.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 2.Falowski SM. Deep Brain Stimulation for Chronic Pain. Curr Pain Headache Rep. 2015 Jul;19(7):27. doi: 10.1007/s11916-015-0504-1. [DOI] [PubMed] [Google Scholar]
- 3.Papka RE, Traurig HH, Klenn P. Paracervical ganglia of the female rat: histochemistry and immunohistochemistry of neurons, SIF cells, and nerve terminals. Am J Anat. 1987 Jul;179(3):243–57. doi: 10.1002/aja.1001790306. [DOI] [PubMed] [Google Scholar]
- 4.Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery. 1987 Dec;21(6):885–93. doi: 10.1227/00006123-198712000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Pereira EA, Boccard SG, Aziz TZ. Deep brain stimulation for pain: distinguishing dorsolateral somesthetic and ventromedial affective targets. Neurosurgery. 2014 Aug;61(Suppl 1):175–81. doi: 10.1227/NEU.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 6.Dostrovsky JO. Role of thalamus in pain. Prog Brain Res. 2000;129:245–57. doi: 10.1016/S0079-6123(00)29018-3. [DOI] [PubMed] [Google Scholar]
- 7.Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991 Mar 15;251(4999):1355–8. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- 8.Ploner M, Gross J, Timmermann L, Schnitzler A. Cortical representation of first and second pain sensation in humans. Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12444–8. doi: 10.1073/pnas.182272899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999 Feb;79(2–3):105–11. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 10.Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999 Jul 06;96(14):7705–9. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones EG. The thalamus. 2. Cambridge ; New York: Cambridge University Press; 2007. [Google Scholar]
- 12.Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001 Feb;24(2):122–6. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- 13.McCormick DA, Feeser HR. Functional implications of burst firing and single spike activity in lateral geniculate relay neurons. Neuroscience. 1990;39(1):103–13. doi: 10.1016/0306-4522(90)90225-s. [DOI] [PubMed] [Google Scholar]
- 14.Le Masson G, Renaud-Le Masson S, Debay D, Bal T. Feedback inhibition controls spike transfer in hybrid thalamic circuits. Nature. 2002 Jun 20;417(6891):854–8. doi: 10.1038/nature00825. [DOI] [PubMed] [Google Scholar]
- 15.Jahnsen H, Llinas R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–26. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huh Y, Bhatt R, Jung D, Shin HS, Cho J. Interactive responses of a thalamic neuron to formalin induced lasting pain in behaving mice. PLoS One. 2012;7(1):e30699. doi: 10.1371/journal.pone.0030699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheong E, Kim C, Choi BJ, Sun M, Shin HS. Thalamic ryanodine receptors are involved in controlling the tonic firing of thalamocortical neurons and inflammatory pain signal processing. J Neurosci. 2011 Jan 26;31(4):1213–8. doi: 10.1523/JNEUROSCI.3203-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha GE, Lee J, Kwak H, Song K, Kwon J, Jung SY, et al. The Ca2+-activated chloride channel anoctamin-2 mediates spike-frequency adaptation and regulates sensory transmission in thalamocortical neurons. Nat Commun. 2016 Dec 19;7:13791. doi: 10.1038/ncomms13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huh Y, Cho J. Discrete pattern of burst stimulation in the ventrobasal thalamus for anti-nociception. PLoS One. 2013;8(6):e67655. doi: 10.1371/journal.pone.0067655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, Park D, Choi S, Lee S, Sun M, Kim C, et al. Thalamic control of visceral nociception mediated by T-type Ca2+ channels. Science. 2003 Oct 3;302(5642):117–9. doi: 10.1126/science.1088886. [DOI] [PubMed] [Google Scholar]
- 21.LeBlanc BW, Cross B, Smith KA, Roach C, Xia J, Chao YC, et al. Thalamic Bursts Downregulate Cortical Theta and Nociceptive Behavior. Sci Rep. 2017 May 30;7(1):2482. doi: 10.1038/s41598-017-02753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris KD, Mrsic-Flogel TD. Cortical connectivity and sensory coding. Nature. 2013 Nov 07;503(7474):51–8. doi: 10.1038/nature12654. [DOI] [PubMed] [Google Scholar]
- 23.Swadlow HA, Gusev AG. The impact of 'bursting' thalamic impulses at a neocortical synapse. Nat Neurosci. 2001 Apr;4(4):402–8. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- 24.Swadlow HA. Thalamocortical control of feed-forward inhibition in awake somatosensory 'barrel' cortex. Philos T Roy Soc B. 2002 Dec 29;357(1428):1717–27. doi: 10.1098/rstb.2002.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010 Dec 15;30(50):16796–808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011 Jan 01;71(1):45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997 Sep;7(6):476–86. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 28.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004 Oct;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 29.Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007 Apr;10(4):462–8. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- 30.Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005 Oct 20;48(2):315–27. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010 Jan 28;65(2):230–45. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003 Nov;90(5):2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- 33.Potter LE, Paylor JW, Suh JS, Tenorio G, Caliaperumal J, Colbourne F, et al. Altered excitatory-inhibitory balance within somatosensory cortex is associated with enhanced plasticity and pain sensitivity in a mouse model of multiple sclerosis. J Neuroinflammation. 2016 Jun 10;13(1):142. doi: 10.1186/s12974-016-0609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cichon J, Blanck TJJ, Gan WB, Yang G. Activation of cortical somatostatin interneurons prevents the development of neuropathic pain. Nat Neurosci. 2017 Aug;20(8):1122–32. doi: 10.1038/nn.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- 36.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005 Jan 20;45(2):201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham JB, McCrum-Gardner E. Power, effect and sample size using GPower: Practical issues for researchers and members of research ethics committees. Evidence Based Midwifery. 2007;5(4):132–6. [Google Scholar]
- 38.Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007 Sep;4(3):S143–56. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- 39.Predicted irradiance values: model based on direct measurements in mammalian brain tissue. Web. http://web.stanford.edu/group/dlab/cgi-bin/graph/chart.php. http://web.stanford.edu/group/dlab/cgi-bin/graph/chart.php.
- 40.Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A. theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res. 2009 Dec;199(3–4):411–21. doi: 10.1007/s00221-009-1961-8. [DOI] [PubMed] [Google Scholar]
- 41.Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, et al. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci. 2011 Jan 26;31(4):1193–203. doi: 10.1523/JNEUROSCI.1379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen ML, Yao L, Boger J, Mercer K, Thompson B, Jiang N. Non-invasive brain stimulation interventions for management of chronic central neuropathic pain: a scoping review protocol. BMJ Open. 2017 Oct 16;7(10):e016002. doi: 10.1136/bmjopen-2017-016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nardone R, Brigo F, Holler Y, Sebastianelli L, Versace V, Saltuari L, et al. Transcranial magnetic stimulation studies in complex regional pain syndrome type I: A review. Acta Neurol Scand. 2018 Feb;137(2):158–64. doi: 10.1111/ane.12852. [DOI] [PubMed] [Google Scholar]
- 44.Kurt E, Henssen D, Steegers M, Staal M, Beese U, Maarrawi J, et al. Motor Cortex Stimulation in Patients Suffering from Chronic Neuropathic Pain: Summary of Expert Meeting and Premeeting Questionnaire, Combined with Literature Review. World Neurosurg. 2017 Dec;108:254–63. doi: 10.1016/j.wneu.2017.08.168. [DOI] [PubMed] [Google Scholar]
- 45.Feustel A, Keitel R, Hornischer R. Significance of the phenolsulphophthalein test for the operative indication for prostatectomy in comparison to the Becher' performance number and the concentration test. Z Urol Nephrol. 1973 Feb;66(2):91–103. [PubMed] [Google Scholar]
- 46.Nizard J, Raoul S, Nguyen JP, Lefaucheur JP. Invasive stimulation therapies for the treatment of refractory pain. Discov Med. 2012 Oct;14(77):237–46. [PubMed] [Google Scholar]
- 47.Butson CR, McIntyre CC. Current steering to control the volume of tissue activated during deep brain stimulation. Brain Stimul. 2008 Jan;1(1):7–15. doi: 10.1016/j.brs.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuimova TF. Trend of work in some Japanese microbiological scientific-research institutes. Mikrobiologiia. 1971 Jul-Aug;40(4):756–9. [PubMed] [Google Scholar]
- 49.George MS, Nahas Z, Kozel FA, Li X, Denslow S, Yamanaka K, et al. Mechanisms and state of the art of transcranial magnetic stimulation. J ECT. 2002 Dec;18(4):170–81. doi: 10.1097/00124509-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Goetz SM, Luber B, Lisanby SH, Murphy DL, Kozyrkov IC, Grill WM, et al. Enhancement of Neuromodulation with Novel Pulse Shapes Generated by Controllable Pulse Parameter Transcranial Magnetic Stimulation. Brain Stimul. 2016 Jan-Feb;9(1):39–47. doi: 10.1016/j.brs.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng. 2011 Aug;8(4):046011. doi: 10.1088/1741-2560/8/4/046011. [DOI] [PubMed] [Google Scholar]
- 52.De Ridder D, Vancamp T, Lenders MW, De Vos CC, Vanneste S. Is preoperative pain duration important in spinal cord stimulation? A comparison between tonic and burst stimulation. Neuromodulation. 2015 Jan;18(1):13–7. doi: 10.1111/ner.12253. discussion 7. [DOI] [PubMed] [Google Scholar]
- 53.Brocker DT, Swan BD, So RQ, Turner DA, Gross RE, Grill WM. Optimized temporal pattern of brain stimulation designed by computational evolution. Sci Transl Med. 2017 Jan 4;9(371) doi: 10.1126/scitranslmed.aah3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang HD, Santaniello S. Closed-loop low-frequency DBS restores thalamocortical relay fidelity in a computational model of the motor loop. Conf Proc IEEE Eng Med Biol Soc. 2017 Jul;2017:1954–7. doi: 10.1109/EMBC.2017.8037232. [DOI] [PubMed] [Google Scholar]
- 55.Cordon I, Nicolas MJ, Arrieta S, Alegre M, Artieda J, Valencia M. Theta-phase closed-loop stimulation induces motor paradoxical responses in the rat model of Parkinson disease. Brain Stimul. 2018 Jan-Feb;11(1):231–8. doi: 10.1016/j.brs.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Russo M, Cousins MJ, Brooker C, Taylor N, Boesel T, Sullivan R, et al. Effective Relief of Pain and Associated Symptoms With Closed-Loop Spinal Cord Stimulation System: Preliminary Results of the Avalon Study. Neuromodulation. 2018 Jan;21(1):38–47. doi: 10.1111/ner.12684. [DOI] [PubMed] [Google Scholar]
- 57.Dmochowski JP, Koessler L, Norcia AM, Bikson M, Parra LC. Optimal use of EEG recordings to target active brain areas with transcranial electrical stimulation. Neuroimage. 2017 Aug 15;157:69–80. doi: 10.1016/j.neuroimage.2017.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edemann-Callesen H, Habelt B, Wieske F, Jackson M, Khadka N, Mattei D, et al. Non-invasive modulation reduces repetitive behavior in a rat model through the sensorimotor cortico-striatal circuit. Transl Psychiatry. 2018 Jan 10;8(1):11. doi: 10.1038/s41398-017-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veerakumar A, Berton O. Cellular mechanisms of deep brain stimulation: activity-dependent focal circuit reprogramming? Curr Opin Behav Sci. 2015 Aug 1;4:48–55. doi: 10.1016/j.cobeha.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frohlich F. Experiments and models of cortical oscillations as a target for noninvasive brain stimulation. Prog Brain Res. 2015;222:41–73. doi: 10.1016/bs.pbr.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 61.Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006 Jun 16;312(5780):1622–7. doi: 10.1126/science.1124593. [DOI] [PubMed] [Google Scholar]
- 62.Boudreau CE, Ferster D. Short-term depression in thalamocortical synapses of cat primary visual cortex. J Neurosci. 2005 Aug 03;25(31):7179–90. doi: 10.1523/JNEUROSCI.1445-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010 Feb 01;518(3):389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000 Apr;3(4):366–71. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 65.Eto K, Wake H, Watanabe M, Ishibashi H, Noda M, Yanagawa Y, et al. Inter-regional contribution of enhanced activity of the primary somatosensory cortex to the anterior cingulate cortex accelerates chronic pain behavior. J Neurosci. 2011 May 25;31(21):7631–6. doi: 10.1523/JNEUROSCI.0946-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eto K, Ishibashi H, Yoshimura T, Watanabe M, Miyamoto A, Ikenaka K, et al. Enhanced GABAergic activity in the mouse primary somatosensory cortex is insufficient to alleviate chronic pain behavior with reduced expression of neuronal potassium-chloride cotransporter. J Neurosci. 2012 Nov 21;32(47):16552–9. doi: 10.1523/JNEUROSCI.2104-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013 May 28;110(22):9130–5. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balmer TS. Perineuronal Nets Enhance the Excitability of Fast-Spiking Neurons. eNeuro. 2016 Jul-Aug;3(4) doi: 10.1523/ENEURO.0112-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975 Nov 21;98(3):417–40. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 70.Rahman A, Lafon B, Bikson M. Multilevel computational models for predicting the cellular effects of noninvasive brain stimulation. Prog Brain Res. 2015;222:25–40. doi: 10.1016/bs.pbr.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, et al. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol. 2013 May 15;591(10):2563–78. doi: 10.1113/jphysiol.2012.247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Dijk KJ, Verhagen R, Chaturvedi A, McIntyre CC, Bour LJ, Heida C, et al. A novel lead design enables selective deep brain stimulation of neural populations in the subthalamic region. J Neural Eng. 2015 Aug;12(4):046003. doi: 10.1088/1741-2560/12/4/046003. [DOI] [PubMed] [Google Scholar]
- 73.McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004 Apr;91(4):1457–69. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- 74.Yoshida K, Horch K. Selective stimulation of peripheral nerve fibers using dual intrafascicular electrodes. IEEE Trans Biomed Eng. 1993 May;40(5):492–4. doi: 10.1109/10.243412. [DOI] [PubMed] [Google Scholar]
- 75.Fang ZP, Mortimer JT. Selective activation of small motor axons by quasi-trapezoidal current pulses. IEEE Trans Biomed Eng. 1991 Feb;38(2):168–74. doi: 10.1109/10.76383. [DOI] [PubMed] [Google Scholar]
- 76.Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J Neurosci. 2010 Nov 10;30(45):15067–79. doi: 10.1523/JNEUROSCI.2059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mix A, Hoppenrath K, Funke K. Reduction in cortical parvalbumin expression due to intermittent theta-burst stimulation correlates with maturation of the perineuronal nets in young rats. Dev Neurobiol. 2015 Jan;75(1):1–11. doi: 10.1002/dneu.22205. [DOI] [PubMed] [Google Scholar]
- 78.Ji RR, Schlaepfer TE, Aizenman CD, Epstein CM, Qiu D, Huang JC, et al. Repetitive transcranial magnetic stimulation activates specific regions in rat brain. Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15635–40. doi: 10.1073/pnas.95.26.15635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zaghi S, Heine N, Fregni F. Brain stimulation for the treatment of pain: A review of costs, clinical effects, and mechanisms of treatment for three different central neuromodulatory approaches. J Pain Manag. 2009 Aug;2(3):339–52. [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen JP, Nizard J, Keravel Y, Lefaucheur JP. Invasive brain stimulation for the treatment of neuropathic pain. Nat Rev Neurol. 2011 Sep 20;7(12):699–709. doi: 10.1038/nrneurol.2011.138. [DOI] [PubMed] [Google Scholar]
- 81.DosSantos MF, Martikainen IK, Nascimento TD, Love TM, DeBoer MD, Schambra HM, et al. Building up analgesia in humans via the endogenous mu-opioid system by combining placebo and active tDCS: a preliminary report. PLoS One. 2014;9(7):e102350. doi: 10.1371/journal.pone.0102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Udupa K, Bahl N, Ni Z, Gunraj C, Mazzella F, Moro E, et al. Cortical Plasticity Induction by Pairing Subthalamic Nucleus Deep-Brain Stimulation and Primary Motor Cortical Transcranial Magnetic Stimulation in Parkinson's Disease. J Neurosci. 2016 Jan 13;36(2):396–404. doi: 10.1523/JNEUROSCI.2499-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giordano J, Bikson M, Kappenman ES, Clark VP, Coslett HB, Hamblin MR, et al. Mechanisms and Effects of Transcranial Direct Current Stimulation. Dose Response. 2017 Jan-Mar;15(1) doi: 10.1177/1559325816685467. 1559325816685467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abbasnia K, Ghanbari A, Abedian M, Ghanbari A, Sharififar S, Azari H. The effects of repetitive transcranial magnetic stimulation on proliferation and differentiation of neural stem cells. Anat Cell Biol. 2015 Jun;48(2):104–13. doi: 10.5115/acb.2015.48.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cullen CL, Young KM. How Does Transcranial Magnetic Stimulation Influence Glial Cells in the Central Nervous System? Front Neural Circuits. 2016;10:26. doi: 10.3389/fncir.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fenoy AJ, Goetz L, Chabardes S, Xia Y. Deep brain stimulation: are astrocytes a key driver behind the scene? CNS Neurosci Ther. 2014 Mar;20(3):191–201. doi: 10.1111/cns.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tawfik VL, Chang SY, Hitti FL, Roberts DW, Leiter JC, Jovanovic S, et al. Deep brain stimulation results in local glutamate and adenosine release: investigation into the role of astrocytes. Neurosurgery. 2010 Aug;67(2):367–75. doi: 10.1227/01.NEU.0000371988.73620.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vedam-Mai V, van Battum EY, Kamphuis W, Feenstra MG, Denys D, Reynolds BA, et al. Deep brain stimulation and the role of astrocytes. Mol Psychiatry. 2012 Feb;17(2):124–31. 15. doi: 10.1038/mp.2011.61. [DOI] [PubMed] [Google Scholar]
- 89.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, et al. Evidence for brain glial activation in chronic pain patients. Brain. 2015 Mar;138(Pt 3):604–15. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008 Oct;21(5):570–9. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kruger L, Light AR, editors. Frontiers in Neuroscience. Boca Raton, FL: 2010. Translational Pain Research: From Mouse to Man. [PubMed] [Google Scholar]
- 92.Barnes WL, Lee WH, Peterchev AV. Approximating transcranial magnetic stimulation with electric stimulation in mouse: a simulation study. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:6129–32. doi: 10.1109/EMBC.2014.6945028. [DOI] [PubMed] [Google Scholar]
- 93.Oashi H, Matsuhashi M, Matsuhashi S. Thymidine diphosphate 4-acetamido-4,6-dideoxyhexoses. IV. Purification and properties of thymidine diphosphate 4-keto-6-deoxy-D-glucose transaminase from Pasteurella pseudotuberculosis. J Biol Chem. 1971 Apr 25;246(8):2325–30. [PubMed] [Google Scholar]
- 94.Estrada C, Tarragon E, Kelley JB, Lopez D, Gil-Martinez AL, Villalba EF, et al. Transcranial Magnetic Stimulation on Rodent Models. CNS Neurol Disord Drug Targets. 2016;15(7):756–64. doi: 10.2174/1871527315666160518125341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.