Figure 3. Therapeutic strategies to target spinal interneuronal (SpIN) plasticity.
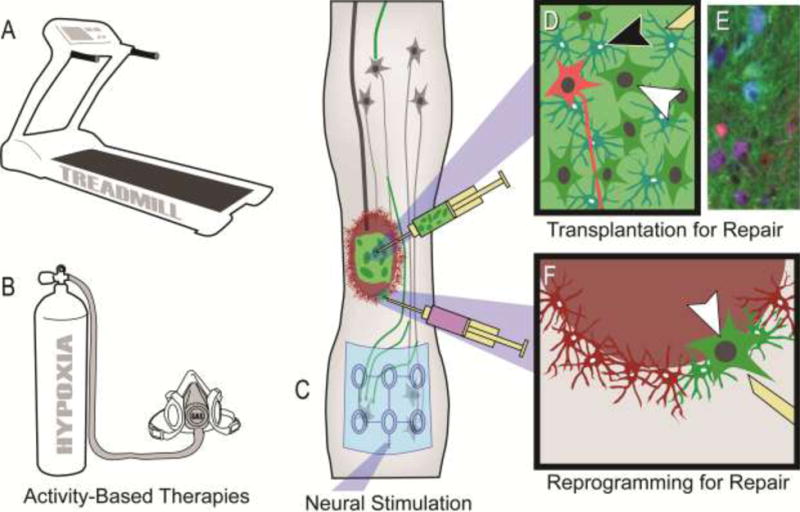
Neuroplasticity within spinal networks can be enhanced by increasing activity within neurons spared by injury or repairing injured neural substrates. Strategies targeting neurons and circuitry spared by injury can typically be divided into 1) activity-based therapies that stimulate activity and afferent feedback to the spinal cord, and 2) more invasive electrical stimulation of central or peripheral neural pathways and neurons. A range of activity-based therapies exist, that can target specific aspects of function, such as locomotor training (A) or respiratory training with intermittent exposure to low-oxygen (hypoxia; B). Neural stimulation can be applied almost anywhere in the periphery, spinal cord (e.g. schematic of an epidural stimulating array electrode in C) or brain. The limitation of such approaches is that they rely on neural pathways spared by the injury. In contrast, some repair strategies, such as cell transplantation (D,E) or in vivo cellular reprogramming (F), can provide new sources of spinal neurons that can form novel pathways and contribute to plasticity. Transplantation of neuronal and glial progenitors (white and black arrowheads in D, respectively) provides the building blocks for repair, and these cells can be tagged to track their distribution (e.g. with a green dye as shown). An important consideration with such repair strategies is the type of neuron being provided. Cellular engineering is now being used to direct the development of donor neuronal progenitors towards specific SpIN subtypes which can be tagged with separate markers (shown in red in D, white arrowhead). An example of recent work using this approach in the injured rat spinal cord is shown in E (one month following transplantation of neuronal lineage-restricted progenitors in blue-cyan, enriched with V2a neurons in red-purple). Image reproduced with permission from [77]. Alternatively, viral injection into a spinal contusion cavity 1 week after mid-cervical injury in the adult rat, can selectively target cells (e.g. astrocytes shown in red in E) and convert them into neurons (converting cells shown in green. Arrowhead in indicates a converted neuron. *Please note that treadmill training for individuals with SCI is usually performed with body-weight support as illustrated in Reier et al. [103]. For respiratory training with intermittent hypoxia individuals are given a small mask that covers their mouth and nose, and intermittently exposed to low-oxygen.
