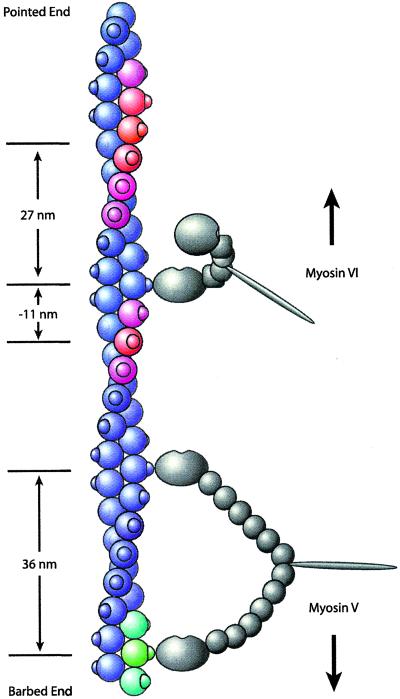
Figure 4.
Model of myosin VI stepping. Myosin V and VI molecules are shown in gray, and the actin filament is shown in color. Knobs on the actin filament indicate stereospecific myosin-binding sites (although the actual binding site is located between two actin monomers, binding is shown on only one monomer for simplicity). Both motors are bound to actin subunits facing directly right. Myosin V (Lower), having six light chains, spans the 36-nm actin pseudorepeat to within ± 1 binding site (green and blue-green). Myosin VI (Upper), having only one light chain and the unique insert (square), cannot. Instead, the bound myosin VI head swings the unbound head to the left side of the actin filament as suggested by the transition from the ADP-bound state to the rigor state in electron microscopy structures (7). The actin subunits shown in red indicate the preferred binding sites of the unbound myosin VI head toward both the pointed end (≈27 nm) and the barbed end (≈11 nm) of actin, corresponding to the modal values seen in the optical trapping experiments. The spread of the color from red to blue reflects the step size histogram. Therefore, nearby actin subunits shown in purple are somewhat less accessible, but steps to these subunits can occur. Subunits shown in blue are relatively inaccessible because their myosin VI-binding sites are on either the right side or the underside of the actin filament. The measured step sizes of myosin VI toward both the pointed end (modal value, 27 nm) and the barbed end (modal value, −11 nm) of actin are a direct result of the accessibility of binding sites on the left side of the actin filament to the unbound myosin VI head.