Abstract
Abnormalities in posttranslational protein modifications (PTMs) that regulate protein targeting, trafficking, synthesis, and function have been implicated in the pathophysiology of schizophrenia. The endoplasmic reticulum (ER) contains specialized machinery that facilitate protein synthesis, ER entry and exit, quality control, and post-translational processing, steps required for protein maturation. Dysregulation of these systems could represent potential mechanisms for abnormalities of neurotransmitter associated proteins in schizophrenia. We hypothesized that expression of ER processing pathways is dysregulated in schizophrenia. We characterized protein and complex expression of essential components from protein folding, ER quality control (ERQC), and ER associated degradation (ERAD) processes in the dorsolateral prefrontal cortex of 12 matched pairs of elderly schizophrenia and comparison subjects. We found increased expression of proteins associated with recognizing and modifying misfolded proteins, including UDP-glucose/glycoprotein glucosyltransferase 2 (UGGT2), ER degradation enhancing alpha-mannosidase like protein 2 (EDEM2), and synoviolin (SYVN1)/HRD1. As SYVN1/HRD1 is a component of the ubiquitin ligase HRD1-SEL1L complex that facilitates ERAD, we immunoprecipitated SEL1L and measured expression of other proteins in this complex. In schizophrenia, SYVN1/HRD1 and OS-9, ERAD promoters, have increased association with SEL1L, while XTP3-B, which can prevent ERAD of substrates, has decreased association. Abnormal expression of proteins associated with ERQC and ERAD suggests dysregulation in ER localized protein processing pathways in schizophrenia. Interestingly, the deficits we found are not in the protein processing machinery itself, but in proteins that recognize and target incompletely or misfolded proteins. These changes may reflect potential mechanisms of abnormal neurotransmitter associated protein expression previously observed in schizophrenia.
Keywords: Postmortem brain, SYVN1/HRD1-SEL1L ubiquitin ligase complex, ERQC, ERAD
Introduction
While the pathophysiology of schizophrenia is not well-understood, many hypotheses focus on abnormalities of neurotransmission in this illness. The dopamine, serotonin, glutamate, and GABA neurotransmitter systems have all been implicated in schizophrenia(Baou et al., 2016; Gonzalez-Burgos and Lewis, 2012; Howes and Kapur, 2009; Nakazawa et al., 2012), and the plurality of abnormalities of neurotransmitter associated proteins suggests dysfunction of cellular processes common to the regulation of these systems. Multiple abnormalities in expression of receptors and transporters associated with glutamate and GABA signaling, including abnormal N-glycosylation, receptor localization, and expression of endoplasmic reticulum (ER)-retention signals suggest dysfunctional processing of neurotransmitter associated proteins in the ER(Bauer et al., 2010; Kristiansen et al., 2010; Mueller et al., 2014; Mueller et al., 2015; Tucholski et al., 2013a; Tucholski et al., 2013b).
The ER is the first compartment in the intracellular secretory pathway, through which the majority of secreted and intramembrane proteins pass (Ellgaard and Helenius, 2003; Vembar and Brodsky, 2008). Protein synthesis, folding, and post-translational processing occur within the ER and are essential for proper protein maturation (Ellgaard and Helenius, 2003; Vembar and Brodsky, 2008). As the initial stage in protein synthesis and processing, the ER contains a highly regulated system for recognizing unfolded and misfolded proteins (Ellgaard and Helenius, 2003; Vembar and Brodsky, 2008). Folding of nascent polypeptide chains in the ER is monitored and regulated by chaperone proteins and folding enzymes, which facilitate the folding and assembly of newly synthesized proteins(Ellgaard and Helenius, 2003; Vembar and Brodsky, 2008). Many newly synthesized proteins are glycosylated, and transfer of N-glycans occurs in a single enzymatic step. The two outermost glucose residues of the N-glycans are rapidly and sequentially removed by mannosyl-oligosaccharide glucosidase (MOGS, also called glucosidase I) and glucosidase II(Araki and Nagata, 2012; Grinna and Robbins, 1979). Glucosidase II is composed of a catalytic subunit, neutral alpha glucosidase AB (GANAB), and a regulatory subunit, glucosidase 2 subunit beta (PRKCSH) (Varki, 2009). The resulting N-glycan isrecognized by the ER lectin-like chaperones calnexin (CNX) and/or calreticulin (CRT), which promote proper folding by protecting glycoproteins from aggregation or premature export from the ER(Araki and Nagata, 2012; Rutkevich and Williams, 2011; Williams, 2006). UDP-glucose/glycoprotein glucosyltransferase (UGGT) senses the folding state of glycoproteins released by CNX/CRT and targets correctly folded and assembled proteins for export to the Golgi(Ellgaard and Helenius, 2003; Vembar and Brodsky, 2008). If proteins are not correctly folded and assembled, UGGT reglucosylates the N-glycan to be reengaged by CNX/CRT (D’Alessio et al., 2010; Solda et al., 2007). Unfolded and terminally misfolded proteins within the CNX/CRT cycle are recognized, modified, and extracted from the ER for degradation by the ubiquitin (UB) proteasome system (UPS)(Ellgaard and Helenius, 2003), by the process of ER associated degradation (ERAD). ERAD glycoprotein substrates are recognized and modified by the lectins EDEM1/2/3, followed by association and modification by substrate recognition complexes including chaperones (GRP78, GRP94), lectins (OS.9, XTP3-B), and reductases(Christianson et al., 2011; Cormier et al., 2009; Maattanen et al., 2010; Ninagawa et al., 2014; Satoh et al., 2010; Vembar and Brodsky, 2008). OS-9 and XTP3-B directly bind with SEL1L, an adaptor protein that is essential for maintaining interaction with the E3 ubiquitin-protein ligase synoviolin (SYVN1; the human homolog of the E3 ligase HRD1 expressed in mice and yeast)(Christianson et al., 2011; Christianson et al., 2008). Substrates targeted to this complex are ubiquitinated by SYVN1/HRD1, a process that facilitates substrate recognition by the proteasome for degradation(MacGurn et al., 2012). The SYVN1/HRD1 UB ligase complex interacts with substrate extraction machinery(Christianson et al., 2008; St Pierre and Nabi, 2012; Vembar and Brodsky, 2008), including the transmembrane proteins derlin-1 and derlin-2 (DERL1/2), which eject substrates from the ER by forming a pore in the membrane(St Pierre and Nabi, 2012; Vembar and Brodsky, 2008). VCP-interacting membrane protein (VIMP) interacts with both DERLs and acts as a recruitment factor for cytosolic VCP-containing complexes (Christianson et al., 2011; Lee et al., 2015). VCP is an AAA-ATPase that is essential for substrate extraction from the ER into the cytosol in an ATP-dependent manner, where they are then degraded by the proteasome (Christianson et al., 2011; Zhong et al., 2004).
ER protein processing, ERQC, and ERAD are particularly important in neurons, and multiple reports suggest that Alzheimer’s disease (AD) (Hoozemans et al., 2009; Hoozemans et al., 2005), Parkinson’s disease (PD) (Hoozemans et al., 2007; Ryu et al., 2002), amyotrophic lateral sclerosis (ALS)(Atkin et al., 2008), Huntington’s disease (HD) (Carnemolla et al., 2009; Duennwald and Lindquist, 2008), and Creutzfeldt Jacob disease (CJD)(Hetz et al., 2003) are all associated with ER stress and associated dysregulation. ER dysfunction has also been implicated in mood disorders and schizophrenia (Bown et al., 2000; Gold et al., 2013; Hunsberger et al., 2011; Nevell et al., 2014; So et al., 2007). To further characterize potential ER-associated abnormalities, we hypothesized that in schizophrenia there is dysregulation of ER protein processing pathways and proteins associated with protein folding, ERQC, and ERAD. This study sought to measure protein and complex expression of essential components of these processes in schizophrenia brain.
Materials and Methods
Subjects
Samples were obtained from the Mount Sinai/Bronx Veterans Administration Medical Center brain collection. Assessment, consent, and postmortem procedures were conducted as required by the Institutional Review Boards of Pilgrim Psychiatric Center, Mount Sinai School of Medicine, and the Bronx Veterans Administration Medical Center. Patients were diagnosed with schizophrenia by two clinicians using DSM-III-R criteria, and had a documented history of psychiatric symptoms before the age of 40, as well as 10 or more years of hospitalization. Criteria for subject exclusion included a history of substance abuse, death by suicide, or coma for greater than 6 hours prior to death. Comparison subjects had no evidence of neuropathology, or signs of neurodegenerative disorders including Alzheimer’s disease at assessment(Funk et al., 2012; Hammond et al., 2010; Mueller et al., 2014; Powchik et al., 1998; Purohit et al., 1998). Whole brains were dissected into 10mm slabs in coronal plane. Grey matter from the dorsolateral prefrontal cortex (DLPFC, Brodmann areas 9/46) was blocked into 1cm cubes from 12 patients with schizophrenia and matched comparison subjects (Table 1 and Supplementary Table S1) and stored at −80°C until use.
Table 1.
Summary of subject demographics
| Comparison | Schizophrenia | |
|---|---|---|
| N | 12 | 12 |
| Age | 73.67 ± 9.04 | 75.83 ± 10.39 |
| Sex | 6M/6F | 6M/6F |
| PMI (hours) | 7.76± 7.54 | 13.58 ± 6.08 |
| Tissue pH | 6.43 ± 0.27 | 6.46 ± 0.20 |
| On/Off Rx | 0/12 | 6/4 |
Abbreviations: Postmortem Interval (PMI); Cause of death (COD); Rx: On = treated with antipsychotic medications at time of death; Off = off antipsychotic medication for ≥ 6 weeks prior to death.
Sample preparation
Tissue samples were reconstituted on ice in 5 mM Tris–HCl pH 7.5, 0.32 mM sucrose with a protease inhibitor tablet and a phosphatase inhibitor tablet (Complete Mini, EDTA-free and PhosSTOP, both from Roche Diagnostics, Mannheim, Germany) using a Power Gen 125 tissue homogenizer (Thermo Fisher Scientific, Rockford, Illinois). Protein concentration was determined using a BCA protein assay kit (Thermo Scientific, Rockford, Illinois). After homogenization, samples were stored at −80°C until use.
SEL1L Co-Immunoprecipitation (Co-IP)
A 300 μg aliquot of each sample homogenate was used for a SEL1L Co-IP using M-280 sheep anti-mouse IgG Dynabeads (Life Technologies, Grand Island, NY). These beads are magnetic, permitting the use of a magnet to separate the supernatant from the beads after each incubation or washing step. To remove non-specific protein binding, a pre-clear step was performed by incubating the sample with 100 μl of beads for 1 hr at 4°C. 100 μl of fresh beads were first washed with cold Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBST) and then blocked with TBST containing 0.1 % BSA for 30 minutes at room temperature (RT) to decrease non-specific binding. Following this, the beads were incubated with rotation at RT for 1 hour with 5 μg of anti-SEL1L antibody (LS Bio, # LS-B2253). Antibody-containing supernatant was then removed and excess antibody washed off with TBST. The antibody-bound beads were incubated rotaing for 1 hour at RT with the pre-cleared sample homogenate from each subject. The supernatant was removed and the beads were washed with TBST to remove unbound protein. Finally, the beads were incubated in 2× loading buffer (0.2 M Tris-hydrochloride with 12% glycerol, 1.5% sodium dodecyl sulfate (SDS), and 0.7% BME) for 10 minutes at 70°C to elute the bound protein off of the beads. The supernatant was then transferred to a new tube and stored at −20°C until further use.
Western Blot Analysis
Target proteins of interest were quantified from schizophrenia and comparison subject pairs using western blot analysis. Samples were diluted in milli-Q water and 6× reducing buffer (4.5% SDS, 15% β-mercaptoethanol (BME), 0.018% bromophenol blue, and 36% glycerol in 170 mM Tris-HCl, pH 6.8) and heated at 70°C for 10 minutes prior to loading. Samples were run in duplicate, with 10 μg of protein loaded in each lane of a 4–12% gradient bis-tris 1.0 mm 17 well gel (Life Technologies). Gel electrophoresis was with a Novex Mini Cell NuPAGE system (Life Technologies, Grand Island, NY) using NuPAGE 2-(N-morpholino) ethanesulfonic acid SDS (MES) running buffer (Life Technologies). After electrophoresis, samples were transferred to 0.45 μm nitrocellulose membranes using a BioRad semi-dry transblotter (Hercules, CA) then briefly rinsed with TBS. Membranes were blocked with Odyssey blocking buffer (927–40000, LI-COR Biosciences, Lincoln, NE) or 5 % Bovine Serum Albumin (BSA) in TBS for 1 hour at room temperature (RT), then incubated overnight at 4°C in primary antisera diluted in Odyssey blocking buffer containing with TBS 0.1% Tween-20 or 5% BSA in TBST (Supplementary Table S2). After washing with TBST, blots were incubated with the appropriate secondary antibody and washed with TBST before being scanned on an Odyssey Infrared Imaging System (LI-COR Biosciences) at a resolution of 169 μm and intensity level of 5. All antibodies were within the range of detection and optimized such that the primary antibody was present in excess.2). VCP antibodies from separate host species (either rabbit or mouse) were used; this was necessary to permit simultaneous quantification of both the protein of interest and VCP as an intralane normalizing control on the same blots.
Data analysis
Image Studio Lite Version 4.0.21 (LiCor) (LiCor,) was used to determine the relative expression of each protein. Integrated signal values of target proteins were normalized to the intralane value of VCP, a ubiquitously expressed protein previously shown to be unchanged in schizophrenia in multiple brain regions(Bauer et al., 2009; Mueller et al., 2014; Stan et al., 2006) while proteins measured from the SEL1L Co-IP were normalized to intralane SEL1L expression. Total VCP expression and SEL1L expression in the Co-IP experiment were not different between schizophrenia and comparison subjects, consistent with previous reports (Bauer et al., 2009; Stan et al., 2006). For each protein, subjects with protein expression below the limit of detection were removed from analysis; no more than 1 subject was removed for any protein of interest. Duplicate values were averaged for each subject and all data were determined to be normally distributed using the D’Agostino-Pearson Omnibus Normality test. Between group comparisons were made by paired Student’s t-tests (α = 0.05). Pearson correlation coefficients (r) (α = 0.05) were used to determine associations between protein expression and subject age, tissue pH, and postmortem interval (PMI). Additionally, potential sex effects were determined by one-way ANOVA. We performed multiple testing corrections using the Benjamini-Hochberg method (Benjamini and Hochberg, 1995) to control the false discovery rate (FDR) and q-values are reported. In this study, we used a fairly high value for a FDR of 0.20 which calculated by [(cutoff p value) × (number of probe sets tested)/(total number of positives)], as appropriate starting points for additional analysis (Miller et al., 2008). Values of FDR in the range of 0.10–0.20 are reasonable in many problems(Genovese et al., 2002). No associations with age, pH, or PMI, or any sex effect was observed for any dependent measure.
Results
Abnormal protein expression of ERQC components in schizophrenia
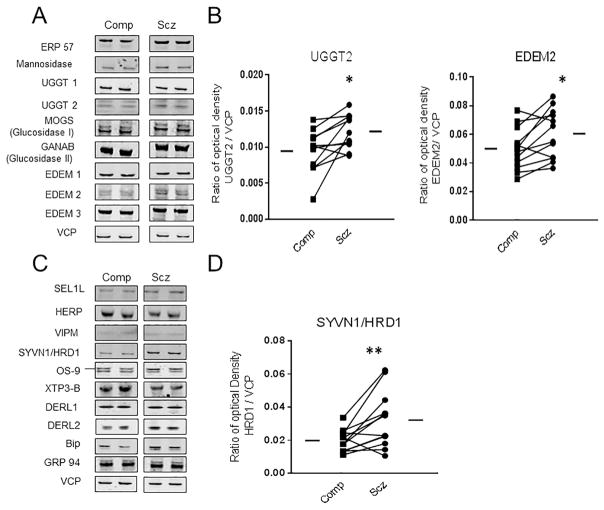
Protein expression of molecular chaperones and protein modifying enzymesfrom the primary protein folding/quality control pathway in the ER was measured in the DLPFC of paired schizophrenia and comparison subjects (Figure 1, Table 2). Significant increases were found in schizophrenia for protein expression of UGGT2 [t(10) = 3.00, p < 0.05] and EDEM2 [t(11) = 2.97, p < 0.05] (Figure 1). No significant differences were observed in the protein expression of other components of this pathway, including MOGS GANAB, Mannosidase I, CNX, CRT, UGGT1, EDEM1, and EDEM3 (Table 2).
Figure 1. Protein expression of protein folding machinery and ER quality control and ER associated degradation components.
Expression of proteins that facilitate protein folding, ER QC and ERAD were measured in the DLPFC of paired schizophrenia and comparison subjects. (A) Representative western blots are shown in paired comparison and schizophrenia subjects. (B) UGGT2 and EDEM2 expression were both significantly increased in schizophrenia. (C) Western blots of assayed ERAD associated proteins from a representative pair of comparison and schizophrenia subjects. (D) SYNV1/HRD1 expression was significantly increased in schizophrenia. **p < 0.01.
Table 2.
Protein expression of protein folding machinery and ER quality control (ERQC) components
| Target Protein | Comparison | Schizophrenia | t | p | q |
|---|---|---|---|---|---|
| ERP57 | 0.022 ± 0.013 | 0.032 ± 0.023 | 1.4 | ||
| Mannosidase | 0.046 ± 0.016 | 0.064 ± 0.032 | 2.0 | ||
| UGGT 1 | 0.38 ± 0.14 | 0.47 ± 0.22 | 1.2 | ||
| UGGT 2 | 0.0095 ± 0.0029 | 0.012 ± 0.007 | 3.0 | 0.012 | 0.04 |
| MOGS Glucosidase I | 0.037 ± 0.010 | 0.047 ± 0.023 | 1.8 | ||
| GANAM Glucosidase II | 0.051 ± 0.015 | 0.065 ± 0.0035 | 1.8 | ||
| EDEM 1 | 1.2 ± 0.41 | 1.3 ± 0.63 | 1.3 | ||
| EDEM 2 | 0.050 ± 0.015 | 0.060 ± 0.017 | 3.0 | 0.013 | 0.016 |
| EDEM 3 | 0.029 ± 0.023 | 0.045 ± 0.037 | 1.3 |
Data are the ratios of optical density values of target proteins divided by optical density of intralane VCP, and reported as means ± s.d. All data were normally distributed and analyzed using paired Student’s t-tests from 12 matched subject pairs. The Benjamini - Hochberg method was used to adjust for multiple testing.
Abnormal protein expression of ERAD components in schizophrenia
UGGT2 and EDEM2 are critical for recognition of terminally misfolded proteins and targeting of these proteins for ERAD. To test whether there is a parallel upregulation in ERAD components in schizophrenia, proteins that recognize ERAD target substrates and facilitate their export from the ER were measured. A significant increase in SYVN1/HRD1 expression [t(11) = 3.11, p < 0.01] was found in schizophrenia, but no significant differences in other components that facilitate recognition (BIP, GRP94, OS-9, and XTP3B) or export (SEL1L, VIMP, HERP, DERL1, and DERL2) of ERAD substrates were found (Figure 1, Table 3).
Table 3.
Protein expression of ER associated degradation (ERAD) components
| Target Protein | Comparison | Schizophrenia | t | p | q |
|---|---|---|---|---|---|
| SEL1 | 0.0094 ± 0.0048 | 0.013 ± 0.001 | 1.4 | ||
| HERP | 0.22 ± 0.01 | 0.22 ± 0.010 | 0.0 | ||
| VIPM | 0.039 ± 0.007 | 0.040 ± 0.0013 | 0.2 | ||
| SYVN1/HRD1 | 0.020 ± 0.007 | 0.032 ± 0.017 | 3.1 | 0.0099 | 0.018 |
| OS-9 | 15 ± 6.2 | 17 ± 5.6 | 1.0 | ||
| XTP3-B | 17 ± 3.5 | 14 ± 2.6 | 0.9 | ||
| DERL1 | 0.46 ± 0.17 | 0.39 ± 0.02 | 1.2 | ||
| DERL2 | 0.40 ± 0.19 | 0.44 ± 0.19 | 0.7 | ||
| Bip | 0.034 ± 0.017 | 0.051 ± 0.044 | 1.4 | ||
| GRP 94 | 0.029 ± 0.008 | 0.040 ± 0.001 | 1.7 |
Data are the ratios of optical density values of target proteins divided by optical density of intralane VCP, and reported as means ± s.d. All data were normally distributed and analyzed using paired Student’s t-tests from 12 matched subject pairs. The Benjamini - Hochberg method was used to adjust for multiple testing
ERAD components are abnormally expressed within the SYVN1/HRD1 complex in schizophrenia
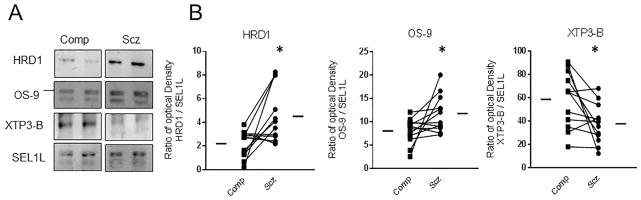
SYVN1/HRD1 is an essential component of a complex that facilitates the extraction and delivery of ERAD-targeted proteins from the ER to the cytosol for degradation by the proteasome. To determine whether increased SYVN1/HRD1 protein expression is associated with abnormal expression of proteins of this complex, co-IPSEL1L, a component of the SYVN1/HRD1 complex, was performed and proteins known to be associated with this complex were measured in this co-IP from the same pairs of schizophrenia and comparison subjects. SYVN1/HRD1 [t(11) = 2.89, p < 0.05] and OS-9 [t(11) = 2.30, p < 0.05] expression were both increased (Figure 2, Table 4) were found. In addition, XTP3-B [t(11) = 2.69, p < 0.05] (Figure 3, Table 4) was decreased in schizophrenia.
Figure 2. Protein expression of components of the SYNV1/HRD1-SEL1L complex expressed in a SEL1L co-immunoprecipitation.
Protein-protein interactions from the SYNV1/HRD1-SEL1L ubiquitin ligase complex were assessed by co-immunoprecipitation (co-IP) of SEL1L followed by western immunoblotting of known binding partners. Western blots of SEL1L co-IP from a representative comparison and schizophrenia pair. (B) SYNV1/HRD1 and OS-9 were significantly increased, whereas XTP3-B was decreased in schizophrenia relative to comparison subjects. *p < 0.05.
Table 4.
Protein expression of components of the HRD1-SEL1L complex expressed in a SEL1L co-immunoprecipitation
| Target Protein | Comparison | Schizophrenia | t | p | q |
|---|---|---|---|---|---|
| SYVN1/HRD1 | 2.2 ± 1.1 | 4.5 ± 2.3 | 2.9 | 0.015 | 0.024 |
| OS-9 | 8.0 ± 2.7 | 12 ± 3.9 | 2.3 | 0.042 | 0.048 |
| XTP3-B | 59 ± 24 | 38 ± 17 | 2.7 | 0.021 | 0.032 |
Data are the ratios of optical density values of target protein divide by optical density of intralane SEL1L, and reported as means ± s.d. All data normally distributed and analyzed using paired Student’s t-tests from 12 matched subject pairs. The Benjamini - Hochberg method was used to adjust for multiple testing
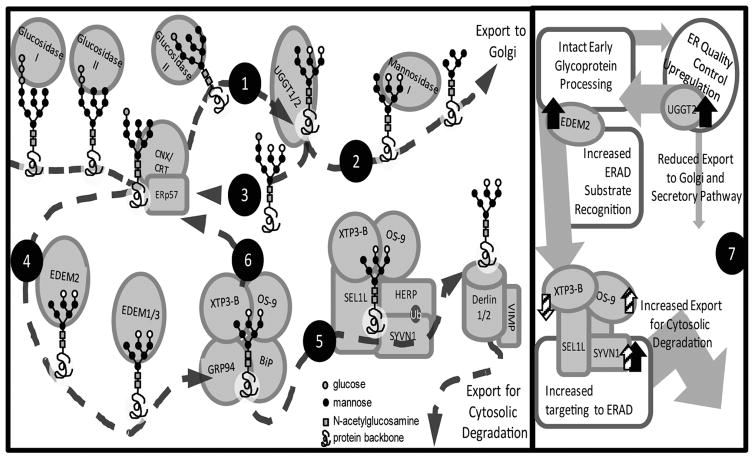
Figure 3. Summary of Protein Folding, Quality Control, and ER Associated Degradation Pathways.
Folding of nascent polypeptide chains in the ER is monitored and regulated by chaperone proteins and folding enzymes, which assist the folding and assembly of newly synthesized proteins. For proteins modified by N-linked glycosylation, the two outermost glucose residues are rapidly removed by glucosidases I & II, allowing the nascent proteins to be picked up by the calreticulin or calnexin (CRT or CNX) folding apparatus and to fold, aided by ERp57, a protein disulfide isomerase that facilitates folding/disulfide bond formation. Glucosidase II then removes another glucose residue, (1) allowing UGGT1 and 2 to bind and assess the protein’s folding status. (2) Properly folded proteins are then modified by ER Mannosidase I and targeted for further processing in the Golgi apparatus, while (3) unfolded/misfolded proteins in are sent back to the CNX/CRT cycle for further processing. (4) Terminally misfolded proteins within this cycle are recognized by the EDEM family of proteins and targeted for ER associated degradation (ERAD). EDEM2 is responsible for the initiation of ERAD targeting, while EDEM1 and 3 perform a subsequent modification that is then recognized by OS-9 (5) OS-9 associates with the SYNV1/HRD1-SEL1L ubiquitin ligase complex, which facilitates ubiquitination of proteins, and leads to the subsequent translocation of terminally misfolded substrates out of the ER, where they are then degraded by the proteasome. (6) XTP3-B also forms complexes with both BiP and the SYNV1/HRD1-SEL1L complex where it may inhibit the degradation of proteins by returning them to the CRT/CNX cycle. (7) In this study we observed increased expression of the ERQC protein UGGT2, and the ERAD-promoting proteins EDEM2 and SYVN1 (denoted by black arrows) in the DLPFC of subjects with schizophrenia. Additionally we observed abnormal expression of SYVN1, XTP3-B, and OS-9 in complex with SEL1L (denoted by striped arrows). Together these findings suggest a pattern of upregulated ERQC and ERAD.
Discussion
Research on the pathophysiology of schizophrenia research has targeted many neurotransmitter and neurochemical systems, resulting in a vast literature reporting abnormal expression of many different classes and families of brain-expressed proteins in this illness. However, abnormalities in myriad different systems may suggest that rather than a disorder of a single neurotransmitter system, schizophrenia may instead be a disturbance of core intracellular processes that underlie regulation of these systems. The ER quality control system is well-placed to have a major impact on proteins associated with multiple neurochemical systems, given that it has a major role in facilities proper processing of proteins and preventing unfolded/misfolded proteins from accumulating in the cell. Protein complexes that facilitate neurotransmission require a high degree of processing and are therefore disproportionally affected by ER dysfunction (Ellgaard and Helenius, 2003). This makes the nervous system particularly sensitive to ER disturbances, as demonstrated by abnormalities in ER stress and quality control systems in neurodegenerative disorders (Hoozemans et al., 2009; Hoozemans et al., 2005, Hoozemans et al., 2007; Ryu et al., 2002, Atkin et al., 2008, Carnemolla et al., 2009; Duennwald and Lindquist, 2008, Hetz et al., 2003). While still relatively unexplored, multiple lines of evidence implicate dysfunction of the ER in schizophrenia (Bown et al., 2000; Gold et al., 2013; Hunsberger et al., 2011; Nevell et al., 2014; So et al., 2007). Therefore, in this study, we investigated ER associated glycoprotein processing machinery by measuring expression of proteins critical for ER glycoprotein folding, processing, and quality control. Enzymes associated with initial modification of glycoproteins (MOGS, GANAB, CNX, CRT, and ERp57) were not abnormally expressed in schizophrenia. However, the fate-determining proteins UGGT2, which recognizes proteins needing further processing and targets them back to the CNX/CRT cycle(Caramelo et al., 2004; Sousa and Parodi, 1995), and EDEM2, an ER-specific mannosidase known to modify terminally misfolded glycoproteins and target them for ERAD(Cormier et al., 2009; Ninagawa et al., 2014; Olivari et al., 2005), were both increased in the DLPFC in schizophrenia.
CNX/CRT are two closely related calcium-binding molecular chaperons and are known to bind to newly synthesized glycoproteins in the ER as part of the folding process, and their function is affected by the concentration of calcium ions in the ER. Multiple studies have revealed a connection between calcium-dependent intercellular pathways and glutamate systems in schizophrenia (Giegling et al., 2010; Lidow, 2003; Park et al., 2015; Snyder and Gao, 2013). Although we have found no differences in expression of CNX or CRT in the study, perturbation of ER calcium homeostasis by glutamate system abnormality could indirectly affect protein folding and glycosylation in the ER lumen.
The UGGT and EDEM protein families are key intracellular quality control points. UGGT proteins recognize the stability of close-to-native folding intermediate states of proteins in the ER and are responsible for either targeting these proteins for export to the Golgi or for reglucosylating proteins that require more processing time in the CNX/CRT cycle(Caramelo et al., 2004; Sousa and Parodi, 1995). Proteins that remain in this cycle for extended periods of time are recognized by the EDEM family, which bind and de-mannosylate terminally misfolded proteins, leading to increased degradation of these proteins(Hosokawa et al., 2001; Olivari et al., 2005). Within each of these protein families, the members appear to have overlapping but non-redundant functions. Early studies of UGGT2 indicated that while an essential gene, it lacks enzymatic activity(D’Alessio et al., 2010; Maattanen et al., 2010), though more recent work has shown conserved UGGT2 glucosylation capacity for at least one substrate and demonstrated kinetic differences between UGGT1 and UGGT2 activity(Takeda et al., 2014). The differential roles of these proteins suggest that upregulation of UGGT2 may underlie abnormal regulation of a specific subset of proteins.
EDEM1, 2, and 3 also appear to have similar, but non-redundant functions. Work by Ninagawa et al. (2014) suggests that, in mammals, EDEM2 is responsible for the initial demannosylation of proteins which targets them from the CNX/CRT cycle to ERAD. After this initial step, EDEM1 and EDEM3 recognize misfolded proteins and edit them further, allowing OS-9 to bind the modified sugar structure and target the protein for degradation(Ninagawa et al., 2014). Upregulation of EDEM2 expression in schizophrenia suggests increased targeting of proteins for ERAD, and previous work showing that overexpression of EDEM2 leads to increased turnover of BACE476(Olivari et al., 2005), a representative glycoprotein, supports this model. Accordingly, we measured protein expression of ERAD components and found increased expression of SYVN1/HRD1, an E3 UB ligase that facilitates targeting of terminally misfolded proteins to the proteasome for degradation(Christianson et al., 2008; Doroudgar et al., 2015; Vembar and Brodsky, 2008).
SYVN1/HRD1 function is dependent upon its association with the structural protein SEL1L, which acts a scaffold for cyclical binding of other elements, and this complex is essential for ERAD of multiple substrates (Mueller et al., 2006). Upregulation of EDEM2, indicates increased targeting of misfolded proteins for degradation, thus we were particularly interested in the association of OS-9 and XTP3-B, glycan binding proteins that are essential for recognition of misfolded proteins(Christianson et al., 2008), with the SYVN1/HRD1-SEL1L complex. In addition to their association with ubiquitin ligases, XTP3-B and OS-9 are also found in complexes responsible for ERAD substrate recognition and processing (Christianson et al., 2011). XTP3-B physically associates with BiP, while OS-9 binds the chaperones GRP94 and BiP (Christianson et al., 2011; Christianson et al., 2008). Interestingly, this interaction appears to be independent of the SYVN1/HRD1-SEL1L complex. Therefore, we immunoprecipitated SEL1L and confirmed the association of OS-9 and XTP3-B with the SYVN1/HRD1-SEL1L complex in our samples. We found increased expression of both SYVN1/HRD1 and OS-9, but decreased expression of XTP3-B in association with SEL1L in schizophrenia. While both OS-9 and XTP3-B have been shown to preferentially bind proteins modified by EDEM1/3, OS-9 association appears to increase ERAD of proteins while XTP3-B has been associated with inhibited degradation of unfolded proteins (Fujimori et al., 2013; Groisman et al., 2011; Mikami et al., 2010; Satoh et al., 2010; Yamaguchi et al., 2010). It has been hypothesized that XTP3-B recognizes unfolded proteins prematurely targeted to ERAD and returns them to the CNX/CRT cycle for further processing (Fujimori et al., 2013). Therefore, the pattern that we found of increased association of OS-9 and decreased association of XTP3-B with SEL1L may reflect upregulation of ERAD through an increased capacity for recognition of ERAD substrates and reduced XTP3-B-dependent retention of proteins. This is consistent with our finding that EDEM2 is increased, resulting in a pattern consistent with increased ERAD in schizophrenia.
Altogether, we found increased expression of various ER protein fate-deciding and degradation-targeting processes. Dysfunction of this system can have a large impact, as between 30–75% of newly synthesized proteins are degraded within 30 minutes, and hetero-oligomeric complex subunits have a particularly poor success rate of maturation (Ellgaard and Helenius, 2003). We have previously shown abnormal content of both NMDA and GABA receptor subunits in the ER (Kristiansen et al., 2010; Mueller et al., 2015) and that GluR2, GluR6, and GABAAR α1 subunits have an abnormal response to endoglycosidase H (Endo-H) Endo-H deglycosylation treatment in schizophrenia(Mueller et al., 2014; Tucholski et al., 2013a; Tucholski et al., 2013b). As Endo-H is specific to immature glycans associated with early ER processing, these findings suggest abnormal glycoprotein processing and expression within the ER. Therefore, we hypothesize that the abnormalities in ER fate-deciding and degradation targeting proteins we observed in the current study may be a mechanism underlying previously observed abnormalities in protein modifications in schizophrenia.
These studies were performed in human postmortem brain with inherent limitations. The subjects analyzed in this study were elderly, therefore these findings may not generalize to younger patients or earlier stages of this illness. Due to the variability in humans compared to the typical laboratory animal, there are many potential confounds that could influence our data. We tested for differences in sex, age, tissue pH, and PMI and confirmed that they had no effect on the proteins measured in this study, in an attempt to control for some of this variance. However, other demographic factors, such as smoking status, were unavailable for this specific cohort and as such are unaccounted for. Additionally, antipsychotic medications have been shown to have a major impact on the brain independent of illness. We compared subjects who were on or off medication, defined as 6 weeks of antipsychotic abstinence prior to death, and found no distinct pattern, though the low number of subjects off medication makes statistical interpretation difficult. Finally, this study focused on protein and complex expression, but not enzymatic activity. Many of the proteins analyzed in this study have important enzymatic functions, and their expression may not completely reflect functional capacity in the cell. Further work to define the consequences of overexpression of UGGT2, EDEM2, and SYVN1/HRD1 in the brain must be performed in order to further elucidate how these findings contribute to schizophrenia pathophysiology.
In summary, this study demonstrates upregulation of proteins associated with ERQC and ERAD machinery in the DLPFC in schizophrenia. We propose our findings may reflect altered ERQC mechanisms in tandem with increased targeting of proteins to ERAD and therefore accelerated and perhaps premature degradation of ER processed proteins. This may be a unifying mechanism contributing to the abnormalities observed in the expression, modification, and function of proteins such as heteromeric neurotransmitter receptors and transporters in schizophrenia.
Supplementary Material
Acknowledgments
ROLE OF FUNDING SOURCES
This work is supported by National Institutes of Health Grant MH53327 (JMW).
The authors would like to thank Dr. Rosalinda Roberts and the Alabama Brain Collection for postmortem cortical samples used in assay development and tests
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
CONTRIBUTORS
Authors PK and JMW and designed the study. PK designed and executed experimental protocols and performed data calculations, statistical analyses, and literature searches, and wrote the first draft of the manuscript and MS helped to manage literature searches and write the draft of the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki K, Nagata K. Protein folding and quality control in the ER. Cold Spring Harbor perspectives in biology. 2012;4(8):a015438. doi: 10.1101/cshperspect.a015438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin JD, Farg MA, Walker AK, McLean C, Tomas D, Horne MK. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiology of disease. 2008;30(3):400–407. doi: 10.1016/j.nbd.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Baou M, Boumba VA, Petrikis P, Rallis G, Vougiouklakis T, Mavreas V. A review of genetic alterations in the serotonin pathway and their correlation with psychotic diseases and response to atypical antipsychotics. Schizophrenia research. 2016;170(1):18–29. doi: 10.1016/j.schres.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophrenia research. 2010;117(1):92–98. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Expression of four housekeeping proteins in elderly patients with schizophrenia. Journal of neural transmission. 2009;116(4):487–491. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown C, Wang JF, MacQueen G, Young LT. Increased temporal cortex ER stress proteins in depressed subjects who died by suicide. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2000;22(3):327–332. doi: 10.1016/S0893-133X(99)00091-3. [DOI] [PubMed] [Google Scholar]
- Caramelo JJ, Castro OA, de Prat-Gay G, Parodi AJ. The endoplasmic reticulum glucosyltransferase recognizes nearly native glycoprotein folding intermediates. The Journal of biological chemistry. 2004;279(44):46280–46285. doi: 10.1074/jbc.M408404200. [DOI] [PubMed] [Google Scholar]
- Carnemolla A, Fossale E, Agostoni E, Michelazzi S, Calligaris R, De Maso L, Del Sal G, MacDonald ME, Persichetti F. Rrs1 is involved in endoplasmic reticulum stress response in Huntington disease. The Journal of biological chemistry. 2009;284(27):18167–18173. doi: 10.1074/jbc.M109.018325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JC, Olzmann JA, Shaler TA, Sowa ME, Bennett EJ, Richter CM, Tyler RE, Greenblatt EJ, Harper JW, Kopito RR. Defining human ERAD networks through an integrative mapping strategy. Nature cell biology. 2011;14(1):93–105. doi: 10.1038/ncb2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JC, Shaler TA, Tyler RE, Kopito RR. OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nature cell biology. 2008;10(3):272–282. doi: 10.1038/ncb1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier JH, Tamura T, Sunryd JC, Hebert DN. EDEM1 recognition and delivery of misfolded proteins to the SEL1L-containing ERAD complex. Molecular cell. 2009;34(5):627–633. doi: 10.1016/j.molcel.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessio C, Caramelo JJ, Parodi AJ. UDP-GlC:glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control. Seminars in cell & developmental biology. 2010;21(5):491–499. doi: 10.1016/j.semcdb.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroudgar S, Volkers M, Thuerauf DJ, Khan M, Mohsin S, Respress JL, Wang W, Gude N, Muller OJ, Wehrens XH, Sussman MA, Glembotski CC. Hrd1 and ER-Associated Protein Degradation, ERAD, are Critical Elements of the Adaptive ER Stress Response in Cardiac Myocytes. Circulation research. 2015;117(6):536–546. doi: 10.1161/CIRCRESAHA.115.306993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duennwald ML, Lindquist S. Impaired ERAD and ER stress are early and specific events in polyglutamine toxicity. Genes & development. 2008;22(23):3308–3319. doi: 10.1101/gad.1673408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nature reviews Molecular cell biology. 2003;4(3):181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Fujimori T, Kamiya Y, Nagata K, Kato K, Hosokawa N. Endoplasmic reticulum lectin XTP3-B inhibits endoplasmic reticulum-associated degradation of a misfolded alpha1-antitrypsin variant. The FEBS journal. 2013;280(6):1563–1575. doi: 10.1111/febs.12157. [DOI] [PubMed] [Google Scholar]
- Funk AJ, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Abnormal activity of the MAPK- and cAMP-associated signaling pathways in frontal cortical areas in postmortem brain in schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37(4):896–905. doi: 10.1038/npp.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Giegling I, Genius J, Benninghoff J, Rujescu D. Genetic findings in schizophrenia patients related to alterations in the intracellular Ca-homeostasis. Progress in neuro-psychopharmacology & biological psychiatry. 2010;34(8):1375–1380. doi: 10.1016/j.pnpbp.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Gold PW, Licinio J, Pavlatou MG. Pathological parainflammation and endoplasmic reticulum stress in depression: potential translational targets through the CNS insulin, klotho and PPAR-gamma systems. Molecular psychiatry. 2013;18(2):154–165. doi: 10.1038/mp.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophrenia bulletin. 2012;38(5):950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinna LS, Robbins PW. Glycoprotein biosynthesis. Rat liver microsomal glucosidases which process oligosaccharides. The Journal of biological chemistry. 1979;254(18):8814–8818. [PubMed] [Google Scholar]
- Groisman B, Shenkman M, Ron E, Lederkremer GZ. Mannose trimming is required for delivery of a glycoprotein from EDEM1 to XTP3-B and to late endoplasmic reticulum-associated degradation steps. The Journal of biological chemistry. 2011;286(2):1292–1300. doi: 10.1074/jbc.M110.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JC, McCullumsmith RE, Funk AJ, Haroutunian V, Meador-Woodruff JH. Evidence for abnormal forward trafficking of AMPA receptors in frontal cortex of elderly patients with schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(10):2110–2119. doi: 10.1038/npp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. The EMBO journal. 2003;22(20):5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJ, van Haastert ES, Eikelenboom P, de Vos RA, Rozemuller JM, Scheper W. Activation of the unfolded protein response in Parkinson’s disease. Biochemical and biophysical research communications. 2007;354(3):707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Eikelenboom P, Scheper W. The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. The American journal of pathology. 2009;174(4):1241–1251. doi: 10.2353/ajpath.2009.080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJ, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P, Scheper W. The unfolded protein response is activated in Alzheimer’s disease. Acta neuropathologica. 2005;110(2):165–172. doi: 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Wada I, Hasegawa K, Yorihuzi T, Tremblay LO, Herscovics A, Nagata K. A novel ER alpha-mannosidase-like protein accelerates ER-associated degradation. EMBO reports. 2001;2(5):415–422. doi: 10.1093/embo-reports/kve084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophrenia bulletin. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsberger JG, Machado-Vieira R, Austin DR, Zarate C, Chuang DM, Chen G, Reed JC, Manji HK. Bax inhibitor 1, a modulator of calcium homeostasis, confers affective resilience. Brain research. 2011;1403:19–27. doi: 10.1016/j.brainres.2011.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostova Z, Tsai YC, Weissman AM. Ubiquitin ligases, critical mediators of endoplasmic reticulum-associated degradation. Seminars in cell & developmental biology. 2007;18(6):770–779. doi: 10.1016/j.semcdb.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Patel SA, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse. 2010;64(7):495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park KJ, Jang JK, Jeon YH, Ko KY, Kwon JH, Lee SR, Kim IY. Selenoprotein S-dependent Selenoprotein K Binding to p97(VCP) Protein Is Essential for Endoplasmic Reticulum-associated Degradation. The Journal of biological chemistry. 2015;290(50):29941–29952. doi: 10.1074/jbc.M115.680215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain research Brain research reviews. 2003;43(1):70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- Maattanen P, Gehring K, Bergeron JJ, Thomas DY. Protein quality control in the ER: the recognition of misfolded proteins. Seminars in cell & developmental biology. 2010;21(5):500–511. doi: 10.1016/j.semcdb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- MacGurn JA, Hsu PC, Emr SD. Ubiquitin and membrane protein turnover: from cradle to grave. Annual review of biochemistry. 2012;81:231–259. doi: 10.1146/annurev-biochem-060210-093619. [DOI] [PubMed] [Google Scholar]
- Mikami K, Yamaguchi D, Tateno H, Hu D, Qin SY, Kawasaki N, Yamada M, Matsumoto N, Hirabayashi J, Ito Y, Yamamoto K. The sugar-binding ability of human OS-9 and its involvement in ER-associated degradation. Glycobiology. 2010;20(3):310–321. doi: 10.1093/glycob/cwp175. [DOI] [PubMed] [Google Scholar]
- Miller JA, Oldham MC, Geschwind DH. A systems level analysis of transcriptional changes in Alzheimer’s disease and normal aging. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28(6):1410–1420. doi: 10.1523/JNEUROSCI.4098-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller B, Lilley BN, Ploegh HL. SEL1L, the homologue of yeast Hrd3p, is involved in protein dislocation from the mammalian ER. The Journal of cell biology. 2006;175(2):261–270. doi: 10.1083/jcb.200605196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TM, Haroutunian V, Meador-Woodruff JH. N-Glycosylation of GABAA receptor subunits is altered in Schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2014;39(3):528–537. doi: 10.1038/npp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TM, Remedies CE, Haroutunian V, Meador-Woodruff JH. Abnormal subcellular localization of GABAA receptor subunits in schizophrenia brain. Translational psychiatry. 2015;5:e612. doi: 10.1038/tp.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62(3):1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevell L, Zhang K, Aiello AE, Koenen K, Galea S, Soliven R, Zhang C, Wildman DE, Uddin M. Elevated systemic expression of ER stress related genes is associated with stress-related mental disorders in the Detroit Neighborhood Health Study. Psychoneuroendocrinology. 2014;43:62–70. doi: 10.1016/j.psyneuen.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninagawa S, Okada T, Sumitomo Y, Kamiya Y, Kato K, Horimoto S, Ishikawa T, Takeda S, Sakuma T, Yamamoto T, Mori K. EDEM2 initiates mammalian glycoprotein ERAD by catalyzing the first mannose trimming step. The Journal of cell biology. 2014;206(3):347–356. doi: 10.1083/jcb.201404075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivari S, Galli C, Alanen H, Ruddock L, Molinari M. A novel stress-induced EDEM variant regulating endoplasmic reticulum-associated glycoprotein degradation. The Journal of biological chemistry. 2005;280(4):2424–2428. doi: 10.1074/jbc.C400534200. [DOI] [PubMed] [Google Scholar]
- Park SJ, Jeong J, Park YU, Park KS, Lee H, Lee N, Kim SM, Kuroda K, Nguyen MD, Kaibuchi K, Park SK. Disrupted-in-schizophrenia-1 (DISC1) Regulates Endoplasmic Reticulum Calcium Dynamics. Scientific reports. 2015;5:8694. doi: 10.1038/srep08694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powchik P, Davidson M, Haroutunian V, Gabriel SM, Purohit DP, Perl DP, Harvey PD, Davis KL. Postmortem studies in schizophrenia. Schizophrenia bulletin. 1998;24(3):325–341. doi: 10.1093/oxfordjournals.schbul.a033330. [DOI] [PubMed] [Google Scholar]
- Purohit DP, Perl DP, Haroutunian V, Powchik P, Davidson M, Davis KL. Alzheimer disease and related neurodegenerative diseases in elderly patients with schizophrenia: a postmortem neuropathologic study of 100 cases. Archives of general psychiatry. 1998;55(3):205–211. doi: 10.1001/archpsyc.55.3.205. [DOI] [PubMed] [Google Scholar]
- Rutkevich LA, Williams DB. Participation of lectin chaperones and thiol oxidoreductases in protein folding within the endoplasmic reticulum. Current opinion in cell biology. 2011;23(2):157–166. doi: 10.1016/j.ceb.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(24):10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Chen Y, Hu D, Hanashima S, Yamamoto K, Yamaguchi Y. Structural basis for oligosaccharide recognition of misfolded glycoproteins by OS-9 in ER-associated degradation. Molecular cell. 2010;40(6):905–916. doi: 10.1016/j.molcel.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Snyder MA, Gao WJ. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Frontiers in cellular neuroscience. 2013;7:31. doi: 10.3389/fncel.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So J, Warsh JJ, Li PP. Impaired endoplasmic reticulum stress response in B-lymphoblasts from patients with bipolar-I disorder. Biological psychiatry. 2007;62(2):141–147. doi: 10.1016/j.biopsych.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Solda T, Galli C, Kaufman RJ, Molinari M. Substrate-specific requirements for UGT1-dependent release from calnexin. Molecular cell. 2007;27(2):238–249. doi: 10.1016/j.molcel.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Sousa M, Parodi AJ. The molecular basis for the recognition of misfolded glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. The EMBO journal. 1995;14(17):4196–4203. doi: 10.1002/j.1460-2075.1995.tb00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pierre P, Nabi IR. The Gp78 ubiquitin ligase: probing endoplasmic reticulum complexity. Protoplasma. 2012;249(Suppl 1):S11–18. doi: 10.1007/s00709-011-0344-8. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: what quality markers matter? Brain research. 2006;1123(1):1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Seko A, Hachisu M, Daikoku S, Izumi M, Koizumi A, Fujikawa K, Kajihara Y, Ito Y. Both isoforms of human UDP-glucose:glycoprotein glucosyltransferase are enzymatically active. Glycobiology. 2014;24(4):344–350. doi: 10.1093/glycob/cwt163. [DOI] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophrenia research. 2013a;146(1–3):177–183. doi: 10.1016/j.schres.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, McMillan LD, Haroutunian V, Meador-Woodruff JH. N-linked glycosylation of cortical N-methyl-D-aspartate and kainate receptor subunits in schizophrenia. Neuroreport. 2013b;24(12):688–691. doi: 10.1097/WNR.0b013e328363bd8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Essentials of glycobiology. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y: 2009. [PubMed] [Google Scholar]
- Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nature reviews Molecular cell biology. 2008;9(12):944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DB. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. Journal of cell science. 2006;119(Pt 4):615–623. doi: 10.1242/jcs.02856. [DOI] [PubMed] [Google Scholar]
- Yamaguchi D, Hu D, Matsumoto N, Yamamoto K. Human XTP3-B binds to alpha1-antitrypsin variant null(Hong Kong) via the C-terminal MRH domain in a glycan-dependent manner. Glycobiology. 2010;20(3):348–355. doi: 10.1093/glycob/cwp182. [DOI] [PubMed] [Google Scholar]
- Zhong X, Shen Y, Ballar P, Apostolou A, Agami R, Fang S. AAA ATPase p97/valosin-containing protein interacts with gp78, a ubiquitin ligase for endoplasmic reticulum-associated degradation. The Journal of biological chemistry. 2004;279(44):45676–45684. doi: 10.1074/jbc.M409034200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.