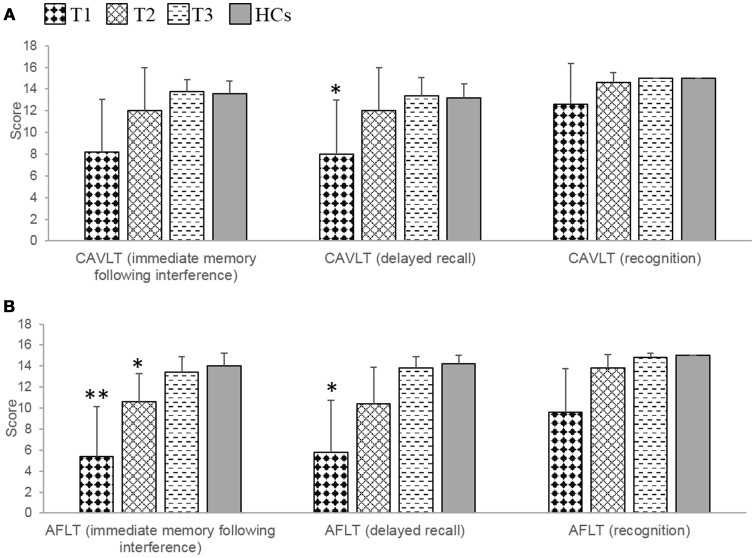
Figure 2.
The results of episodic memory in the patients with anti-NMDAR encephalitis and health controls (HCs). (A) The patients showed marginally significant verbal episodic memory impairments at T1, and no obvious damage at T2 and T3 during the recovery period. (B) The patients showed significant or marginally significant non-verbal episodic memory impairments at T1 and T2, and no obvious damage at T3 during the recovery period. CAVLT, Chinese version of verbal learning test. AFLT, Aggie figures learning test. T1 = 1 to 2 months following initial immunotherapy. T2 = 6 months following initial immunotherapy. T3 = 11 to 12 months following initial immunotherapy. The two-sample t-test between the patients and health controls (HCs) at three time points. **p < 0.05/3; *p < 0.1/3 (Bonferroni correction).