Figure 5.
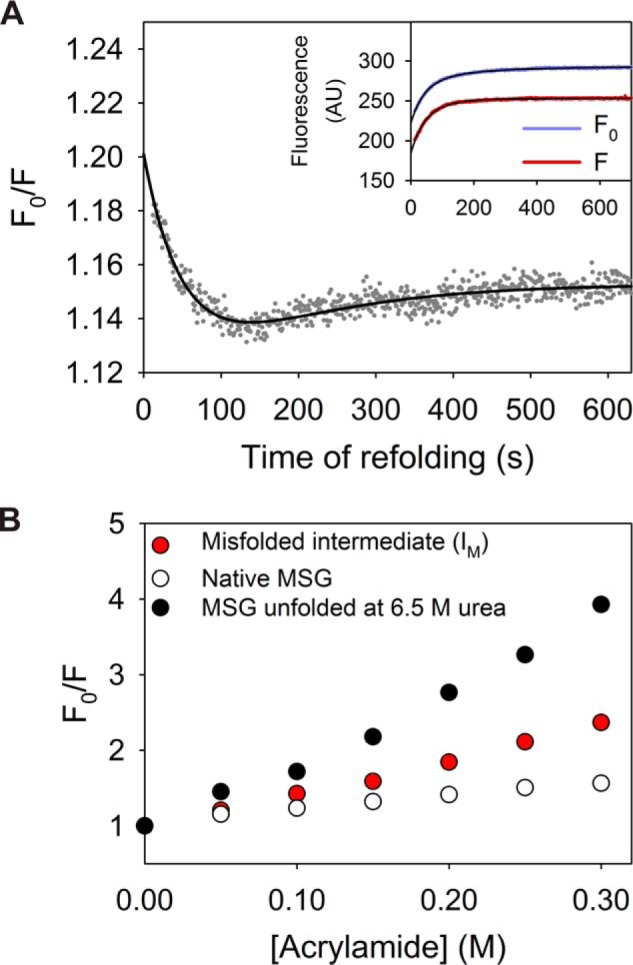
Refolding kinetics of MSG probed by the accessibility of its Trp residues to acrylamide. A, single-jump refolding kinetics (to 1 m urea) were probed by the ratio of Trp fluorescence intensities without and with 0.05 m acrylamide (F0/F). The drop of F0/F to 1.19 (from 1.45 for the unfolded state) within dead time of mixing indicates a collapse of the unfolded polypeptide. The inset represents refolding traces probed by Trp fluorescence in absence (purple) and presence (red) of quencher for reference. B, Stern-Volmer plot representation for comparison of structural compactness of IM (red), as compared with native (white) and unfolded (black) protein at 6.5 m urea.
