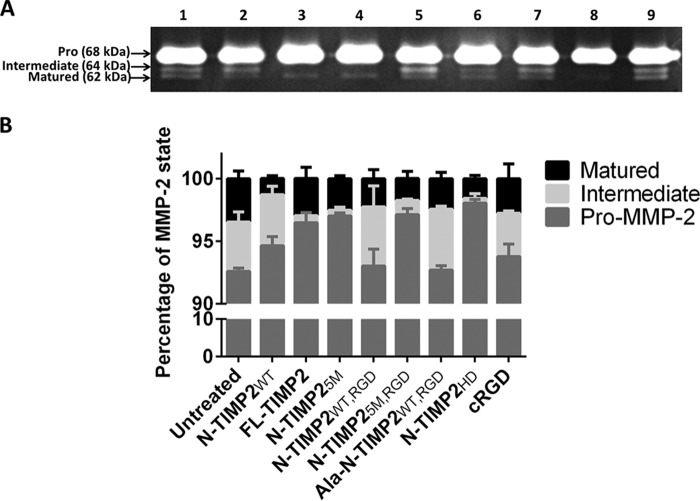
Figure 3.
Inhibition of pro-MMP-2 activation by N-TIMP2 variants. A, gelatin zymography gel results. The U87MG supernatant was loaded on gelatin zymography gel. Lane 1 represents untreated U87MG cells, and lanes 2–8, respectively, represent treatment with 100 nm of the following N-TIMP2 variants: N-TIMP2WT; FL-TIMP2; N-TIMP25M; N-TIMP2WT, RGD; N-TIMP25M, RGD; Ala–N-TIMP2WT, RGD; and N-TIMP2HD. Lane 9 represents treatment with cRGD (100 nm). B, quantification of the percentage of each MMP-2 form, i.e. pro/intermediate/matured (band intensity of each MMP-2 state, i.e. pro-MMP-2 (68 kDa), intermediate MMP-2 (64 kDa), and matured MMP-2 (62 kDa), was divided by the total band intensity of all the MMP-2 forms). Error bars represent S.E. Pro-MMP-2 processing and the total amount of the catalytically active MMP-2 were significantly inhibited by N-TIMP2WT (p < 0.05), FL-TIMP2 (p < 0.01), and N-TIMP5M, N-TIMP25M, RGD, and N-TIMP2HD (p < 0.001). Processing of the intermediate form was significantly inhibited by Ala–N-TIMP2WT, RGD (p < 0.05). The amount of the matured form of MMP-2 was significantly reduced by N-TIMP25M and N-TIMP25M, RGD (p < 0.05), N-TIMP2HD (p < 0.01), and N-TIMP2WT (p < 0.001). Statistical analysis was performed by Student's t test compared with the untreated control; n = 3.