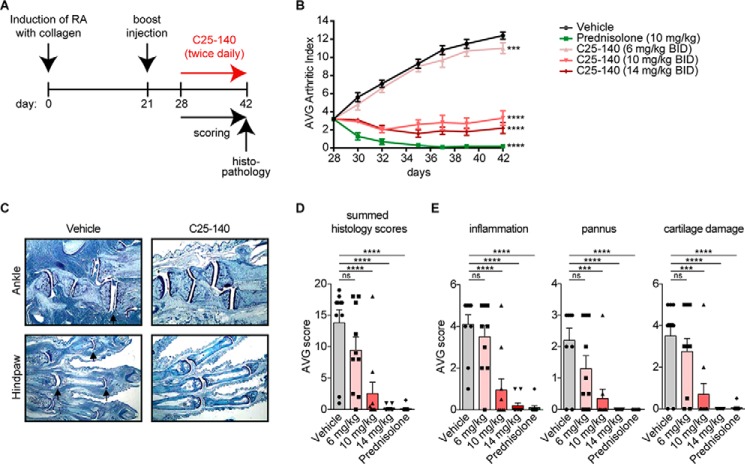
Figure 7.
C25-140 ameliorates symptoms of RA in a preclinical mouse model. A, study design of a CIA preclinical mouse model for RA (n = 10/group). RA was induced by collagen application at day 0 and a booster injection at day 21. At day 28, C25-140 was intraperitoneally applied twice daily for 14 days. Mice were scored throughout the study, and limbs were collected at day 14 of treatment for histopathology. B, the average arthritic index was determined for each group to analyze efficacy of treatment. C25-140 and the control prednisolone significantly reduced the arthritic index; error bars, S.E.; two-way ANOVA test (Sidak's multiple-comparison test). C, in histopathology analyses, mice were scored for hematoxylin and eosin staining of sections for histopathology (ankle and hind paw). C25-140 at 14 mg/kg ameliorates disease outcome; more histology images are shown in Fig. S7. D, summed histology score. E, inflammation, pannus, and cartilage damage. All quantified parameters demonstrate a dose-dependent improvement of RA symptoms after C25-140 treatment; error bars, S.E.; one-way ANOVA test (Sidak's multiple-comparison test); more quantification of histology data is shown in Fig. S8; ns, not significant; ***, p < 0.001; ****, p < 0.0001; BID, twice daily.