Scheme 1.
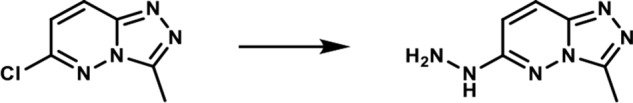
1-(3-Methyl-[1,2,4]-triazolo-[4,3-b]-pyridazin-6-yl)-hydrazine. A round-bottomed flask was charged with 6-chloro-3-methyl-[1,2,4]-triazolo-[4,3-b]-pyridazine (2.5 g, 0.015 mol) and ethanol (35 ml). To the resulting solution was added the hydrazine hydrate (2.6 ml, 0.053 mol). The reaction mixture was then heated at 80 °C for 3 h. After cooling to room temperature, the formed product slurry was filtered, washed with little ethanol, and air-dried to afford 2.43 g (98%) of the desired product as a white solid. 1H NMR (400 MHz, DMSO-d6): δ 8.46 (br s, 1H), 7.86 (d, J = 10 Hz; 1H), 6.78 (d, J = 10 Hz; 1H), 4.28 (br s, 2H), 2.55 (s, 3H); LC-MS (ESI+) m/z: [M + H]+ Calcd. for C6H9N6 165.17 found 165.20, tR = 0.17 min.
