Scheme 4.
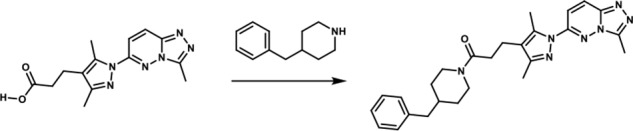
1-(4-Benzylpiperidin-1-yl)-3-(3,5-dimethyl-1-(3-methyl-[1,2,4]-triazolo-[4,3-b]-pyridazin-6-yl)-1H-pyrazol-4-yl)-propan-1-one. To a round-bottomed flask filled with a solution of 3-(3,5-dimethyl-1-(3-methyl-[1,2,4]-triazolo-[4,3-b]-pyridazin-6-yl)-1H-pyrazol-4-yl)-propanoic acid (50 mg, 0.17 mmol), in dimethylformamide (3 ml), were added 4-benzylpiperidine (0.06 ml, 0.33 mmol), HATU (68.52 mg, 0.18 mmol)), and triethyl amine (0.04 ml, 0.306 mmol). The resulting reaction mixture was stirred at room temperature until complete consumption of the starting material (17 h) was observed. It was then diluted with water (15 ml) and extracted with ethyl acetate (3 × 10 ml). The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo. The product was purified by flash chromatography eluting with a linear gradient of ethyl acetate/methanol 100:00 → 50:50 as eluent. The combination of the appropriate fractions yielded 55 mg (70%) of the title product: 1H NMR (400 MHz, DMSO-d6): δ 8.4 (d, J = 10 Hz, 1H), 7.88 (d, J = 10 Hz, 1H), 7.33–7.09 (br m, 3H), 7.10–6.99 (br m, 2H), 4.39 (br d, 1H), 3.76 (br d, 1H), 2.86 (t, 1H), 2.71–2.61 (br m, 6H), 2.59 (s, 3H), 2.47–2.14 (br m, 4H), 2.24 (s, 3H), 1.77–1.63 (br m, 1H), 1.59–1.43 (br m, 2H), 0.99–0.73 (br m, 2H); LC-MS (ESI+) m/z: [M + H]+ Calcd. for C26H32N7O 458.58 found 458.61, tR = 1.16 min.
