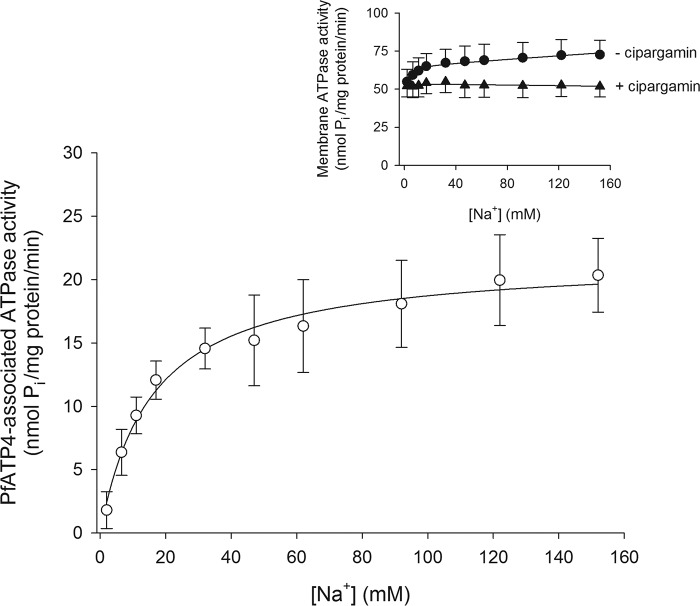
Figure 4.
[Na+] dependence of PfATP4-associated ATPase activity. The PfATP4-associated ATPase activity (white circles) was calculated as described under “Experimental procedures” using the total ATPase activity measured in the presence of 500 nm cipargamin (inset, black triangles) and that measured in the absence of cipargamin (inset, black circles). The data were obtained with Dd2 parasites and are shown as the mean (± S.D.) from eight independent experiments, each performed on different days with different membrane preparations. For reasons of clarity, only upward error bars are shown for the data obtained in the absence of cipargamin (inset), and only downward error bars are shown for the data obtained in the presence of cipargamin (inset). The PfATP4-associated ATPase activity data were fitted with the Michaelis-Menten equation (PfATP4-associated ATPase activity = Vmax × [Na+]/([Na+] + Km(Na+))), yielding a Km(Na+) of 16.1 ± 3.8 mm and a Vmax of 21.8 ± 3.8 nmol/min/mg of protein (mean ± S.D.).