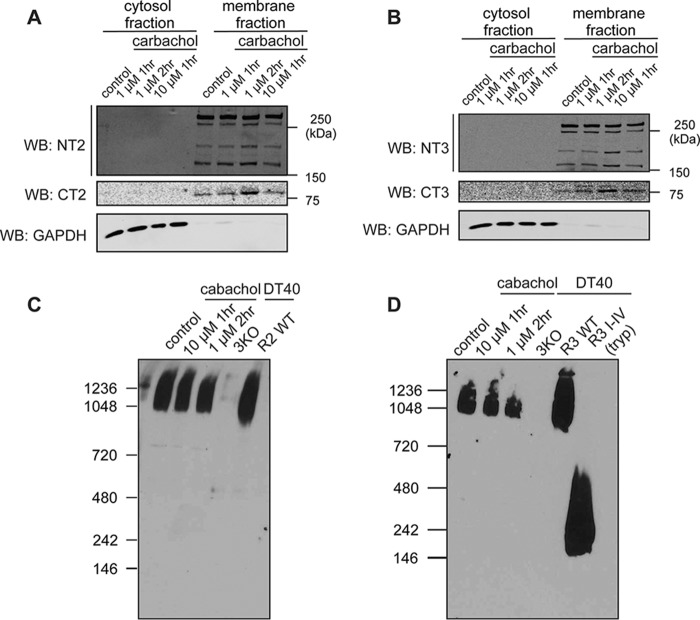
Figure 6.
R2 and R3 retain a tetrameric architecture and ER membrane localization after receptor fragmentation. A–D, isolated rat PAC were incubated with various supramaximal concentrations of carbachol for different amounts of time, followed by membrane fractionation. Full-length receptors, the N-terminal receptor fragments, and the C-terminal receptor fragments all remained membrane-associated for both R2 (A) and R3 (B). Further, native gel analysis showed that fragmented R2 and R3 migrated at the same molecular weight as full-length receptors, indicating that R2 and R3 retain a tetrameric architecture after receptor fragmentation (C and D). R3 I-V represents the migration pattern of the dissociated monomeric N-terminal fragment. Each experiment was repeated three times. WB, Western blot; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.