Abstract
Background and Aims
Excess selenium (Se) is toxic to plants, but relatively little is known about the regulatory mechanism of plant Se tolerance. This study explored the role of the TPS22 gene in Se tolerance in Arabidopsis thaliana.
Methods
Arabidopsis wild type and XVE mutant seeds were grown on half-strength MS media containing Na2SeO3 for screening of the Se-tolerant mutant tps22. The XVE T-DNA-tagged genomic sequence in tps22 was identified by TAIL-PCR. The TPS22 gene was transformed into the mutant tps22 and wild type plants using the flower infiltration method. Wild type, tps22 mutant and transgenic seedlings were cultivated on vertical plates for phenotype analysis, physiological index measurement and gene expression analysis.
Key Results
We identified an Arabidopsis Se-tolerant mutant tps22 from the XVE pool lines, and cloned the gene which encodes the terpenoid synthase (TPS22). TPS22 was downregulated by Se stress, and loss-of-function of TPS22 resulted in decreased Se accumulation and enhanced Se tolerance; by contrast, overexpression of TPS22 showed similar traits to the wild type under Se stress. Further analysis revealed that TPS22 mediated Se tolerance through reduction of Se uptake and activation of metabolism detoxification, which decreased transcription of high-affinity transporters PHT1;1, PHT1;8 and PHT1;9 and significantly increased transcription of selenocysteine methyltransferase (SMT), respectively. Moreover, loss-of-function of TPS22 resulted in reduced cytokinin level and repression of cytokinin signalling components AHK3 and AHK4, and upregulation of ARR3, ARR15 and ARR16. Exogenous cytokinin increased transcription of PHT1;1, PHT2;1 and SMT and decreased Se tolerance of the tps22 mutant. In addition, enhanced Se resistance of the tps22 mutant was associated with glutathione (GSH).
Conclusions
Se stress downregulated TPS22, which reduced endogenous cytokinin level, and then affected the key factors of Se uptake and metabolism detoxification. This cascade of events resulted in reduced Se accumulation and enhanced Se tolerance.
Keywords: TPS22, selenium tolerance, cytokinin, selenium uptake, selenium detoxification, Arabidopsis thaliana
INTRODUCTION
Selenium (Se) is a micronutrient for many organisms but is also toxic at higher levels, and both Se deficiency and Se toxicity are problems worldwide (Pilon-Smits, 2015; Pilon-Smits et al., 2017). Selenate and selenite are the predominant form of Se in soils, and plants absorb them by different mechanisms (Zhu et al., 2009; Huang et al., 2015). Selenate is absorbed by the sulfate transporter, and is metabolized through the sulfur (S) assimilation pathway due to the similar chemical properties between Se and S (Terry et al., 2000; Cabannes et al., 2011; Liu et al., 2016). Selenite is absorbed by the phosphate (Pi) transporters (Li et al., 2008; Zhang et al., 2014; Schiavon and Pilon-Smits, 2017). In Arabidopsis, at least five phylogenetically distinct classes of integral membrane proteins possess Pi transport activity. The PHT1 genes, PHT1;1–PHT1;9, encode high-affinity plasma membrane-localized Pi transporters, which mediate Pi import across the plasma membrane and catalyse Pi/H+ symport activity. All PHT1 genes, except PHT1;6, are predominantly expressed in root tissues. They mediate external Pi uptake, which represents the primary and crucial step in plant Pi acquisition (Remy et al., 2012). The PHT2;1 gene encodes a low-affinity Pi transporter located in the chloroplast inner envelope membrane that mediates Pi translocation within the aerial parts of the plant and influences whole-plant Pi allocation (Daram et al., 1999; Versaw and Harrison, 2002; Remy et al., 2012). Selenite is absorbed by roots and then converted to organic Se to transport to the shoots. In the chloroplast, selenate is reduced to selenite, and then through a variety of complex chemical reactions to generate selenocysteine (SeCys), selenomethionine (SeMet) and other forms of organic Se (Gabel-Jensen & Gammelgaard, 2010; Banuelos et al., 2011; Pilon-Smits, 2015). Se toxicity in plants has been attributed to the formation of non-specific selenoproteins and oxidative stress (Van Hoewyk, 2013). When seleno amino acids inadvertently become incorporated into proteins, replacing Cys and Met, this impairs protein function and thus results in toxicity (Stadtman, 1990; Pilon-Smits, 2015; Gupta and Gupta, 2017). Se non-accumulator plants which accumulate less than 100 mg Se/kg d. wt, e.g. grasses and crops, can metabolize SeMet into volatile dimethylselenide (DMSe) (Terry et al., 2000; Gupta and Gupta, 2017). DMSe was found to be almost 600 times less toxic than inorganic Se compounds (McConnell and Portman, 1952; Wilber, 1980). The production of DMSe is important for non-accumulators to divert potentially toxic SeMet to the significantly less toxic DMSe (Pilon-Smits et al., 2017). Se hyperaccumulator plants accumulate more than 1000 mg Se/kg d. wt, e.g. Astragalus and Stanleya, and thrive well in Se-rich soil (Pilon-Smits et al., 2017). They have methylated forms of SeCys and SeMet. SeCys is methylated to form MetSeCys by selenocysteine methyltransferase (SMT), and finally becomes volatile dimethyldiselenide (DMDSe) (Sors et al., 2005; Pilon-Smits, 2015). DMSe is the main volatile Se compound in non-accumulators, while DMDSe is primarily produced in hyperaccumulators (Pilon-Smits and Le Duc, 2009; Pilon-Smits et al., 2017). Aside from volatilization, another Se detoxification mechanism in plants is the breakdown of SeCys into elemental Se and alanine by selenocysteine lyase (SL) (Van Hoewyk et al., 2005; Van Hoewyk, 2013; Pilon-Smits, 2015). Selenocysteine lyases are analogous to NifS-like Cys desulfurase proteins characterized in Arabidopsis (Pilon-Smits et al., 2002; Ye et al., 2005). The overexpression of AtCpNifS can enhance Se tolerance and accumulation in Arabidopsis (Van Hoewyk et al., 2005; Van Hoewyk, 2013).
Molecular and biochemical studies of non-accumulator plants revealed that plant hormones play an important role in Se defence responses. It was found that genes involved in the ethylene and jasmonic acid pathways were up-regulated by Se in Arabidopsis (Tamaoki et al., 2008a, b). These phytohormones are enhanced via signal pathways of reactive oxygen species (ROS) and then act in a cooperative or antagonistic manner to induce stress responsive genes and Se uptake and metabolic genes (Pilon-Smits et al., 2017; Schiavon and Pilon-Smits, 2017). Previous research also proposed that selenite-induced H2O2 mitigates a selenite-defensive response (Tamaoki et al., 2008b; Lehotai et al., 2012; Van Hoewyk, 2013; Jiang et al., 2016). In addition, Se affects cytokinin (CK) levels in primary roots (Pilon-Smits et al., 2017), while nitric oxide (NO) represses CK signalling (Feng et al. 2013). The higher levels of NO improved Se tolerance in the Arabidopsis mutant gsnor1-3 (Lehotai et al., 2011, 2012).
Some key genes involved in Se tolerance have been cloned and analysed. The overexpression of ATP sulfurylase leads to selenate tolerance (Pilon-Smits et al., 1999). The overexpression of the Se binding protein gene (SBP1) in Arabidopsis can increase resistance to selenite (Agalou et al., 2005; Hugouvieux et al., 2009). However, our understanding of the molecular mechanisms of Se tolerance is far from complete. In this study, we identified an Arabidopsis Se-tolerant mutant tps22 from XVE-tagged T-DNA insertion lines (Zhang et al., 2005) and cloned the corresponding gene which encodes the terpenoid synthase (TPS22). In plants, terpene synthases (TPSs) are responsible for the synthesis of a large class of terpene compounds consisting of a five-carbon isoprene-building unit. Some hormones, for example CKs, are made up by terpenes (Chen et al., 2011; Falara et al., 2011; Tholl and Lee, 2011). Our study demonstrated that CK is involved in TPS22-medaited Se tolerance and aids in the search for the mechanism of Se detoxification and tolerance in plants.
MATERIALS AND METHODS
Plant materials, growth conditions and treatments
The plant materials used in this study included wild-type (WT) Arabidopsis thaliana (L.) Heynh Columbia-0 (Col-0), tps22 mutant, transgenic plants (TPS22-complementary plant COM1; TPS22-overexpressing plant OE1) and ipt1 3 5 7 mutant (Miyawaki et al., 2006). The tps22 mutant was screened from the XVE-tagged T-DNA insertion lines (Zhang et al., 2005). Seeds of the ipt1 3 5 7 quadruple mutant were kindly provided by Dr Dongwei Di.
For phenotype analysis, seeds of WT, mutant or transgenic plants were germinated and grown vertically on half-strength (½) MS (Murashige and Skoog, 1962) media, sodium selenite (Na2SeO3), β-oestradiol (ES), 6-benzylaminopurine (BA) or buthionine sulfoximine (BSO; Sigma). The plates were stored for 3 d in the dark at 4°°C and then placed in a growth chamber maintained at 22 °C and 65 % humidity with a light intensity of 100 μmol m−2 s−1 and a 16-h day length. After the indicated days of growth, plants were sampled for root growth assays and measurement of fresh weight. In addition, the seeds were sown in pots of peat soil/vermiculite/perlite (3: 9: 0.5) presoaked with plant nutrient solution and grown at 22 °C and 65 % humidity with a light intensity of 100 μmol m−2 s−1 and a 16-h day length.
Wild-type, tps22 mutant and transgenic plants were grown on ½ MS media with or without 30 μm Na2SeO3, 0.01 μm BA or 0.1 mM BSO. After the indicated days of growth, seedlings were sampled for Se, Pi, CK and GSH content, GPX activity, ROS level and gene expression analysis. To investigate the organ-specific RNA level of TPS22, the roots, rosette leaves, cauline leaves, stems, inflorescence and siliques were obtained from WT plants grown in soil for 5 weeks.
Screening, identification of mutant and genetic analysis
The tps22 mutant was screened as previously reported (Jiang et al., 2016). WT and XVE mutant seeds were grown on ½ MS media for 7 d and transferred to ½ MS media containing 10 μm β-oestradiol and 45 μm Na2SeO3. Putative mutant seedlings that had a longer root length were transferred to soil to produce seeds.
The XVE T-DNA-tagged genomic sequence in tps22 was identified by TAIL-PCR (Liu et al., 1995; Zuo et al., 2000) and DNA sequencing. The tps22 mutant was crossed with the WT Col-0, and the F1 seedlings were allowed to self-pollinate. F1 and F2 seedlings were then scored for the mutation trait.
Generation of TPS22-complementary and TPS22-overexpressing transgenic lines
Total RNA was extracted from seedlings using Trizol reagent (Invitrogen) according to the manufacturer’s protocol and used to synthesize cDNA. The amplification reactions of the TPS22 gene were performed in 20-μL volumes containing 2 µL of cDNA, 0.4 µL of each primer, 10 µL of PCR SuperMix and 7.2 µL of ddH2O. The PCR thermocycling programme used was as follows: initial denaturation of 10 min at 95 °C; followed by 36 cycles of 30 s at 94 °C, 30 s at 48 °C and 2 min at 72 °C; and a final extension reaction of 10 min at 72 °C. TPS22 was fused to pCAMBIA1301 using the BstEII and NcoI site of the pCAMBIA1301 vector. The 35S::TPS22 construct was introduced into Agrobacterium tumefaciens strain GV3101, which was transformed into the mutant tps22 and WT plants using the flower infiltration method (Clough and Bent, 1998). The transcripts of TPS22-complementary and TPS22-overexpressing lines were detected by semi-quantitative reverse transcriptase PCR (RT-PCR). Total RNA was extracted from 2-week-old seedlings grown on ½ MS with or without 30 μm Na2SeO3. The primers used are listed in Supplementary Data Table S1.
RNA extraction, RT-PCR and quantitative real-time RT-PCR (qRT-PCR) analysis
Total RNA was extracted with Trizol reagent (Invitrogen) according to the manufacturer’s protocol and used to synthesize cDNA. RT-PCR was performed as previously described (Jiang et al., 2016). qRT-PCR was performed according to the instructions provided for the Bio-Rad iCycler iQ system (Bio-Rad), using platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) as previously described (Jiang et al., 2016). The primers used are listed in Table S1. Each sample was quantified at least in triplicate and normalized using ACTIN11 as an internal control. Significant differences between the samples were evaluated by Student’s t-test using delta Ct values (Yuan et al., 2006).
Measurement of Se content
WT, tps22 mutant and transgenic plants were each rinsed with 1 mm CaSO4 and deionized water three times to eliminate any external Se, and then sampled for determination of Se content according to the method described by Liu et al. (2016). Plant tissues were digested with 5 mL HNO3 overnight and then the sample solutions were transferred into a 50-mL volumetric flask with 0.1 m HNO3. The digested samples were diluted with 3 % HCl and analysed using an atomic fluorescence spectrometry detector (AFS-3100).
Measurement of total Pi content
WT and tps22 mutant plants were rinsed in distilled water, blotted dry and ground in liquid nitrogen. The ground tissues were homogenized in 1 % glacial acetic acid and centrifuged at 12 000 g for 5 min. Aliquots of the supernatant solution were assayed for Pi content analysis. The total Pi content in the samples was quantified using a phosphomolybdate colorimetric assay as described previously (Ames, 1966; Versaw and Harrison, 2002).
Measurement of CK content
The extraction and purification of CK was similar to the method described by Sun et al. (2003). In brief, WT, tps22 mutant and transgenic seedlings were ground with 80 % (v/v) methanol. The homogenate was centrifuged at 5000 g for 10 min and the supernatant was collected for passage through a SEP-pak C-18 cartridge to remove the pigments and other lipophilic impurities. The filtrate was collected for CK content analysis. The CK content was determined using a plant CK ELISA Kit (Shanghai Sangon Biotech) following the manufacturer’s instructions.
Measurement of GSH content
WT and tps22 mutant seedlings were ground in liquid nitrogen and extracted in 1 mL of 5 % trichloroacetic acid. The homogenate was centrifuged at 15 000 g for 10 min. The supernatant was collected. Glutathione was measured based on the glutathione reductase-dependent reduction of 5,50-dithiobis (2-nitro-benzoic acid) (DTNB), monitored at 412 nm (Noctor and Foyer, 1998). The reaction mixture containing 10 μL supernatant, 0.1 mL of 0.2 m Na2HPO4 (pH 7.5), 10 mm EDTA, 10 μL of 10 mm NADPH, 10 μL of 12 mm DTNB and 60 μL of water was incubated for 5 min, and the absorbance was recorded at 412 nm. GSH content was expressed in μmol g–1 f. wt.
Analysis of GPX activity
WT, tps22 mutant and transgenic seedlings were ground in liquid nitrogen and extracted in 50 mm sodium phosphate buffer (pH 7.0), containing 1 mm EDTA and 2 % (w/v) polyvinylpyrrolidone. The ground tissues were centrifuged at 12 000 g for 10 min. The supernatant was collected for GPX activity analysis according to the method of Tappel et al. (1978). GPX activity was tested based on the decrease of NADPH at 340 nm. Enzyme activity U is defined as the amount of NADPH (µmol) reduced per minute.
Determination of ROS
WT and tps22 mutant plants were grown on ½ MS media for 7 d and then transplanted in media supplemented with or without 30 μm Na2SeO3 for the indicated times (12, 24 h). ROS were detected using 2′,7′-dichlorofluorescin diacetate (H2DCFDA, a fluorescent dye for ROS) assays according to the method of Xie et al. (2011). The details were described by Jiang et al. (2017). ROS were determinated by using a TCS-SP2 laser scanning confocal microscope (LSCM, Leica Lasertechnik). Fluorescence was expressed as relative fluorescence units using Leica Confocal Software 2.5.
Statistical analysis
Experimental data are given as the means ± s.e. of three replicated groups. Statistical significance is determined by ANOVA combined with a post hoc test, and significant differences (P < 0.05) are indicated by different lowercase letters.
RESULTS
Isolation of Se-resistance mutant
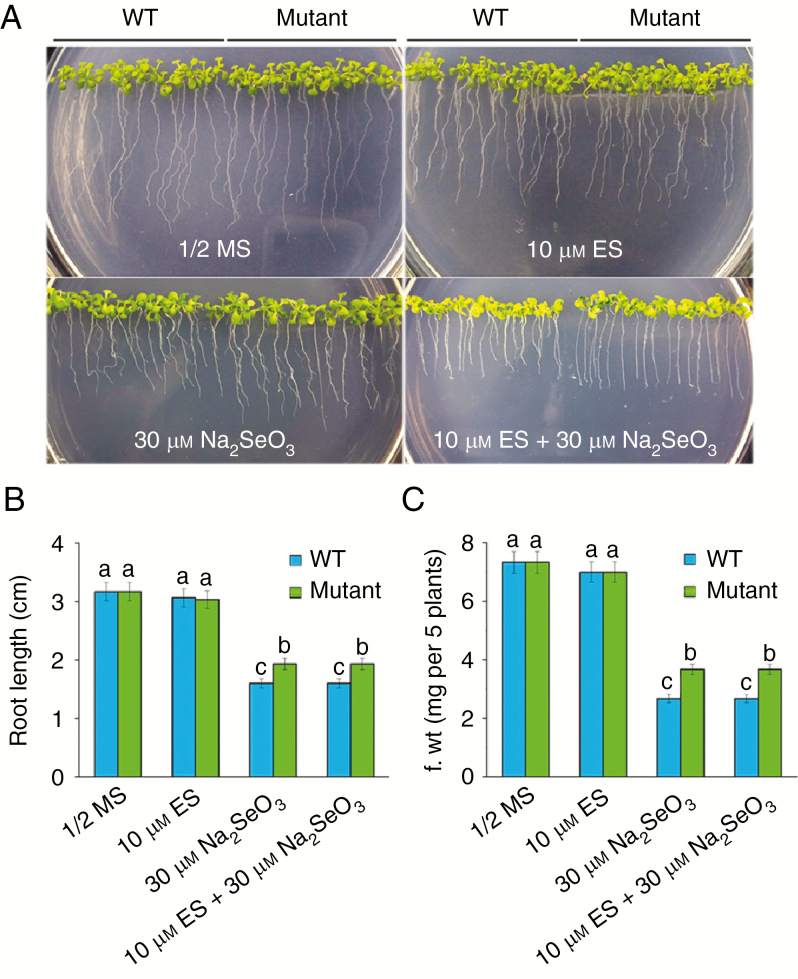
To screen for Se-resistance mutants, we completed a genetic screen (Jiang et al., 2016) and isolated two putative mutants from the XVE-tagged T-DNA insertion lines (Zhang et al., 2005). One of the mutants was chosen for further analysis. To test their Se tolerance, WT and mutant seeds were germinated and grown vertically on ½ MS media with or without 30 μm Na2SeO3 and 10 μm β-oestradiol for 3 weeks. There were no significant differences between the WT and the mutant plants that were grown on ½ MS media; however, when 30 μm Na2SeO3 was added to the ½ MS media with or without 10 μm β-oestradiol, the mutant seedlings were more resistant to Se than WT seedlings (Fig. 1A). Quantitative analyses confirmed that the root length and fresh weight of the mutant were significantly (P < 0.05) higher than those of the WT grown on Se-containing media with or without 10 μm β-oestradiol (Fig. 1B, C). These results suggest that the increased tolerance to Se in the mutant is independent of β-oestradiol. In addition, there were no significant differences between the WT and the mutant under soil cultivation for different periods, except 8 weeks (Fig. S1).
Fig. 1.
Isolation of the Se-resistance mutant. (A) Arabidopsis wild-type (WT) and Se-resistance mutant plants grown on ½ MS media in the absence or presence of 10 μm β-oestradiol (ES) or 30 μm Na2SeO3 for 3 weeks. (B,C) Root length (B) and fresh weight (C) of the plants described in A. Data are the mean ± s.e. of three independent replicates. Significant differences (P < 0.05) are indicated by different lowercase letters.
Genetic analysis of the Se-resistance mutant showed that the F1 plants all exhibited the WT phenotype (Fig. S2). The F2 seedings that resulted from the crosses showed a 3: 1 segregation ratio of the mutant to the WT (Table 1) (χ2 test, P > 0.05). These results indicate that the Se-resistance mutant is a monogenic recessive nuclear mutation.
Table 1.
Genetic analysis of the Se-resistance mutant
| Cross | Progeny | Total | Phenotype | χ2 | |
|---|---|---|---|---|---|
| + | — | ||||
| Mutant | F 1 | 30 | 30 | 0 | |
| ×Col-0 | F 2 | 900 | 654 | 246 | 2.49 |
(+) wild-type; (–) mutant; χ2 was calculated based on an expected ratio of 3: 1 (wild-type/mutant).
Loss-of-function of TPS22 is responsible for the increased Se tolerance
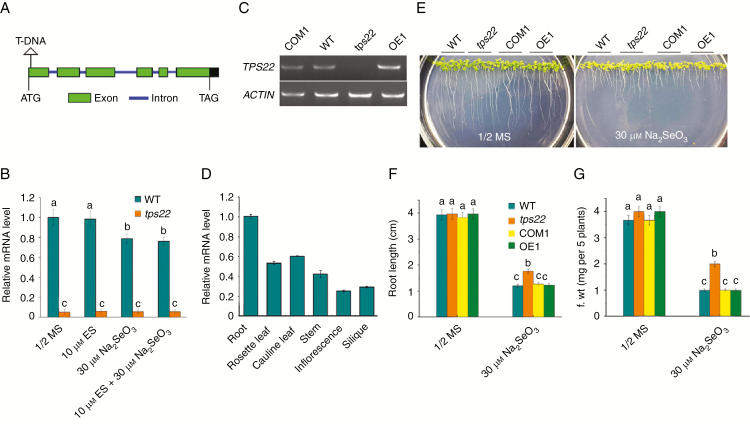
We identified a single T-DNA insertion in the Se-resistance mutant using thermal asymmetric interlaced PCR (TAIL-PCR). The results indicate that T-DNA was inserted into the first exon downstream of the ATG start codon of the gene (At1g33750) (Fig. 2A), previously known as TPS22 (Ro et al., 2006). Therefore, this Se-resistance mutant was designated as tps22. We examined expression levels of the TPS22 gene in the WT and tps22 mutant. TPS22 transcriptions in WT plants were detected and downregulated under Se stress, whereas transcription levels of TPS22 were much lower (Fig. 2B) or not detected (Fig. 2C) in the tps22 mutant in the absence or presence of Se. This suggests that the enhanced Se tolerance in tps22 might be caused by loss-of-function of TPS22. The organ-specific expression pattern of TPS22 by qRT-PCR analysis indicates that TPS22 had a higher expression in the roots and a lower expression in the inflorescences and siliques (Fig. 2D).
Fig. 2.
Cloning of the TPS22 gene. (A) A schematic of the T-DNA insertion site in TPS22. (B) qRT-PCR analysis of TPS22 gene transcription levels in 2-week-old seedlings of the WT and tps22. ACTIN11 was used as the internal control. (C) RT-PCR analysis of the TPS22 gene transcriptions in 2-week-old seedlings of the WT, tps22, and TPS22-complementary (COM1) and TPS22-overexpressing (OE1) transgenic lines. ACTIN11 (ACTIN) was used as a loading control. (D) qRT-PCR analysis of TPS22 transcripts in different tissues of WT plants. RNA was isolated from roots, rosette leaves, cauline leaves, stems, inflorescence and siliques of the 5-week-old WT plants. ACTIN11 was used as the internal control. (E) Phenotypes of WT, tps22, COM1 and OE1 plants grown on ½ MS media with or without 30 μm Na2SeO3 for 2 weeks. (F, G) Root length (F) and fresh weight (G) of the plants described in E. Data are the mean ± s.e. of three independent replicates. Significant differences (P < 0.05) are indicated by different lowercase letters.
To confirm that the enhanced Se tolerance in tps22 was due to a loss-of-function mutant of TPS22, we generated transgenic TPS22-complementary and TPS22-overexpressing plants under the strong CaMV 35S promoter. Complement and overexpression of TPS22 were verified by RT-PCR analysis (Fig. 2C). To test their Se tolerance, seeds of WT and transgenic plants were germinated and grown vertically on ½ MS media with or without 30 μm Na2SeO3 for 2 weeks. As shown in Fig. 2E, the complementary plants COM1 restored the growth state of the WT plants, and the OE1 plants showed similar traits to the WT plants. Quantitative analyses confirmed that the root length and the fresh weight of WT, COM1 and OE1 plants were similar and decreased more than those of the tps22 mutant under Na2SeO3 (Fig. 2F, G).
TPS22 mediates Se tolerance through reduction of Se uptake and activation of metabolism detoxification
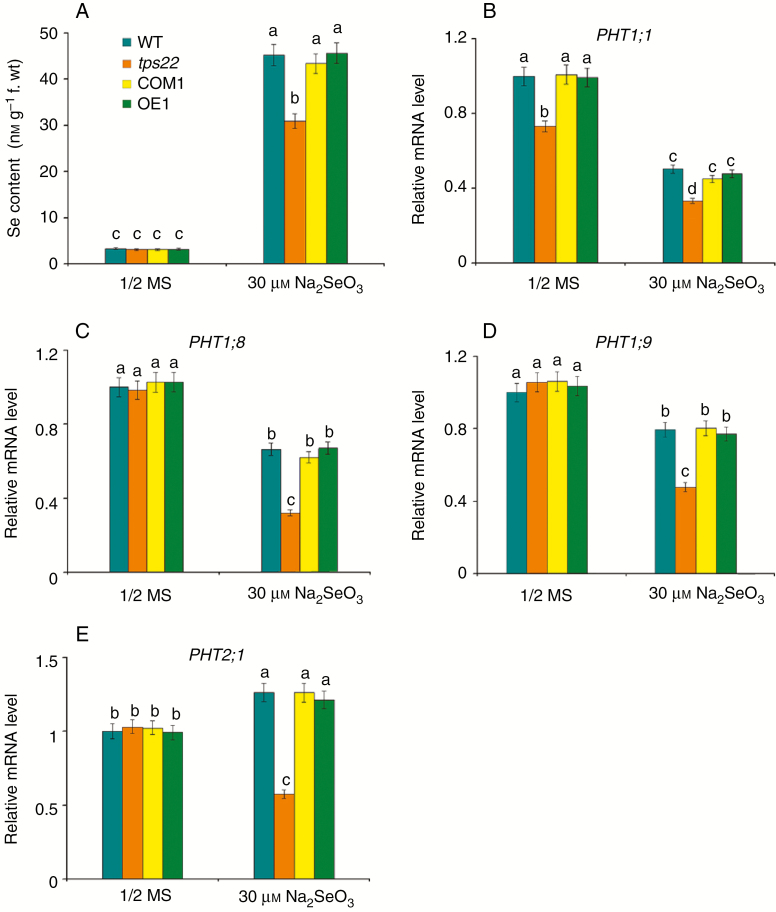
To test whether TPS22-mediated Se tolerance is associated with a change of Se content, we determined the Se content in WT, tps22 and transgenic plants subjected to Se stress and found that Se content in tps22 seedlings was significantly lower than that in the WT, COM1 and OE1 (Fig. 3A), suggesting that enhanced Se resistance in tps22 might be associated with decreased Se content.
Fig. 3.
Loss-of-function of TPS22 resulted in reduced Se accumulation. (A) Se contents in WT, tps22, COM1and OE1 plants grown on ½ MS media with or without 30 μm Na2SeO3 for 2 weeks. (B–E) qRT-PCR analysis of genes involved in Se uptake, including PHT1;1 (B), PHT1;8 (C), PHT1;9 (D) and PHT2;1 (E). WT, tps22 and transgenic lines were grown vertically on ½ MS media with or without 30 μm Na2SeO3 for 2 weeks, and then their mRNAs were isolated for qRT-PCR analysis. ACTIN11 was used as the internal control. Data are the mean ± s.e. of three independent replicates. Significant differences (P < 0.05) between parameters are indicated by different lowercase letters.
Previous research has shown that selenite uptake is mediated by phosphate transporters (Li et al., 2008; Zhang et al., 2014; Schiavon and Pilon-Smits, 2017). Therefore, we investigated the expression of Pi transporters PHT1;1, PHT1;8, PHT1;9 and PHT2;1 in WT, tps22 and transgenic seedlings under Se stress. PHT1;1 transcription level in the tps22 mutant was significantly lower than that in WT in the absence or presence of Na2SeO3 (Fig. 3B). No significant differences in transcription levels of PHT1;8, PHT1;9 and PHT2;1 were observed between the WT and the tps22 mutant grown on ½ MS media, but significantly lower transcription levels of these transporters were detected in the tps22 mutant than in the WT under 30 μm Na2SeO3 stress, and transgenic lines were similar to WT (Fig. 3C–E). In addition, we measured total Pi content in WT and tps22 mutant plants under Se stress, and the result showed Pi content in the tps22 mutant was lower (Fig. S3). These results demonstrate that the lower expression level of these transporters reduces Se uptake and accumulation to contribute to Se tolerance.
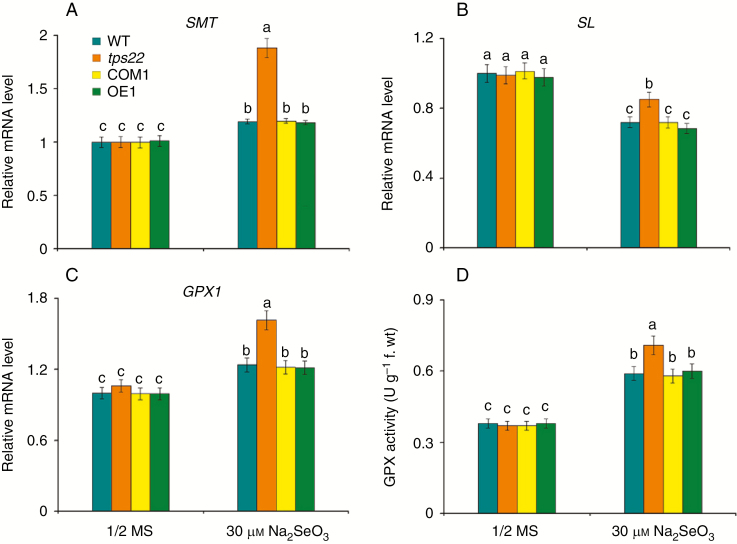
SMT plays a crucial role in Se detoxification and its high efficiency in methylation of selenocysteine prevents the non-specific incorporation of selenoamino acids into proteins (Lyi et al., 2005; Cakir and Ari, 2013). SL metabolizes selenocysteine into Se and alanine. Here, we detected significantly higher transcription levels of SMT in tps22 (Fig. 4A), while the transcription level of SL was less reduced in the tps22 mutant than in the WT treated with 30 μm Na2SeO3 (Fig. 4B), suggesting that the tps22 mutant has a stronger ability to remove the toxic effect of Se.
Fig. 4.
Loss-of-function of TPS22 increased Se metabolism detoxification ability. (A–C) qRT-PCR analysis of genes involved in Se metabolism detoxification, including SMT (A), SL (B) and GPX1 (C). WT, tps22 and transgenic lines were grown vertically on ½ MS media with or without 30 μm Na2SeO3 for 2 weeks, and then their mRNAs were isolated for qRT-PCR analysis. ACTIN11 was used as the internal control. (D) GPX activity of WT, tps22, COM1 and OE1 plants subjected to Se for 2 weeks. Data are the mean ± s.e. of three independent replicates. Significant differences (P < 0.05) between parameters are indicated by different lowercase letters.
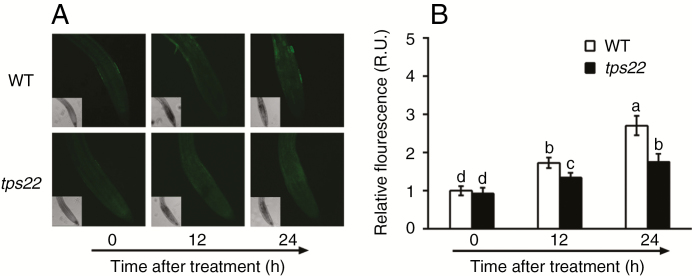
The GPX1 gene encodes Se-dependent glutathione peroxidase (GPX), an ROS scavenger in plants (Stadtman, 1990; Van Hoewyk, 2013). A significantly higher transcript level of GPX1 was detected in tps22 plants than in WT plants under Se stress (Fig. 4C). GPX activity of the tps22 mutant improved more compared to that of the WT and transgenic lines (Fig. 4D). In addition, we also measured ROS accumulation in the WT and mutant plants under Na2SeO3 stress. A significantly lower increase of fluorescence was detected in tps22 plants under Na2SeO3 treatment for 12 h, especially for 24 h (Fig. 5), in agreement with the higher level of GPX1 and GPX activity of the tps22 mutant.
Fig. 5.
ROS production induced by Se. (A) ROS production in seedling root tips of the WT and tps22 mutant with 30 μm Na2SeO3 treatment for 12 and 24 h. Scale bar = 50 μm. (B) Relative fluorescence units (R.U.) of ROS in root tips. WT and tps22 plants were grown vertically on ½ MS media for 7 d and treated with 30 μm Na2SeO3 for 12 and 24 h. The roots were immediately infiltrated with 20 μm H2DCFDA fluorescent probe, and ROS were detected by laser scanning confocal microscopy. Data are the mean ± s.e. of three independent replicates. Significant differences (P < 0.05) are indicated by different lowercase letters.
Together, the above results suggest that TPS22 mediates Se tolerance through reduction of Se uptake and activation of metabolism detoxification.
Cytokinin is involved in the regulation of TPS22-mediated Se tolerance
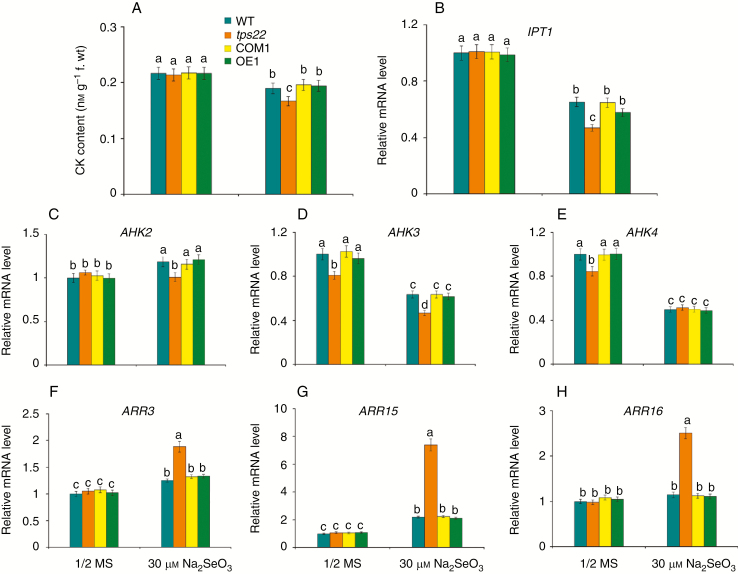
CKs are mainly synthesized in roots and made up by terpenes which are synthesized by TPSs, including TPS22 (Kamada-Nobusada and Sakakibara, 2009; Falara et al., 2011). Thus, we measured CK levels in WT, tps22 and transgenic plants in response to Se stress. As shown in Fig. 6A, CK content in tps22 plants was lower than that in the WT, COM1 and OE1 under Se stress. Accordingly, a significantly lower transcript level of isopentenyl transferase (IPT), which catalyses the rate-limiting step of CK biosynthesis, was detected in mutant seedlings than in WT and transgenic plants (Fig. 6B). In Arabidopsis, CK signalling involves a multistep two-component signalling system comprising CK receptor histidine kinases (AHK2, AHK3 and AHK4), histidine phosphotransfer proteins (AHP1–5) and response regulators (type A and type B ARRs) (Kieber and Schaller, 2014). We found that even without Se treatment, transcript levels of AHK3 and AHK4 were lower in tps22 than those in the WT. Addition of 30 μm Na2SeO3 to the medium reduced AHK3 and AHK4 transcription in the tps22, WT and transgenic seedlings, and increased AHK2 transcription in WT and transgenic plants but not in tps22 plants (Fig. 6C–E). The type-A ARRs are primary response genes for the CK signalling pathway because their expression is rapidly induced as a negative feedback loop (Muller and Sheen, 2007; Kieber and Schaller, 2014). As expected, we detected significantly higher ARR3, ARR15 and ARR16 transcript levels in the tps22 than in WT and transgenic plants in response to Se stress (Fig. 6F–H). These results suggest that CK, acting through the two-component signalling pathway, might be involved in the regulation of TPS22-mediated Se tolerance.
Fig. 6.
Cytokinin (CK) is involved in TPS22-mediated Se tolerance. (A) CK contents in WT, tps22, COM1 and OE1 plants grown on ½ MS media with or without 30 μm Na2SeO3 for 2 weeks. (B–H) qRT-PCR analysis of genes involved in CK biosynthesis and CK signalling, including IPT1 (B), AHK2 (C), AHK3 (D), AHK4 (E), ARR3(F), ARR15(G) and ARR16 (H). WT, tps22 and transgenic lines were grown vertically on ½ MS media with or without 30 μm Na2SeO3 for 2 weeks, and then their mRNAs were isolated for qRT-PCR analysis. ACTIN11 was used as the internal control. Data are the mean ± s.e. of three independent replicates. Significant differences (P < 0.05) between parameters are indicated by different lowercase letters.
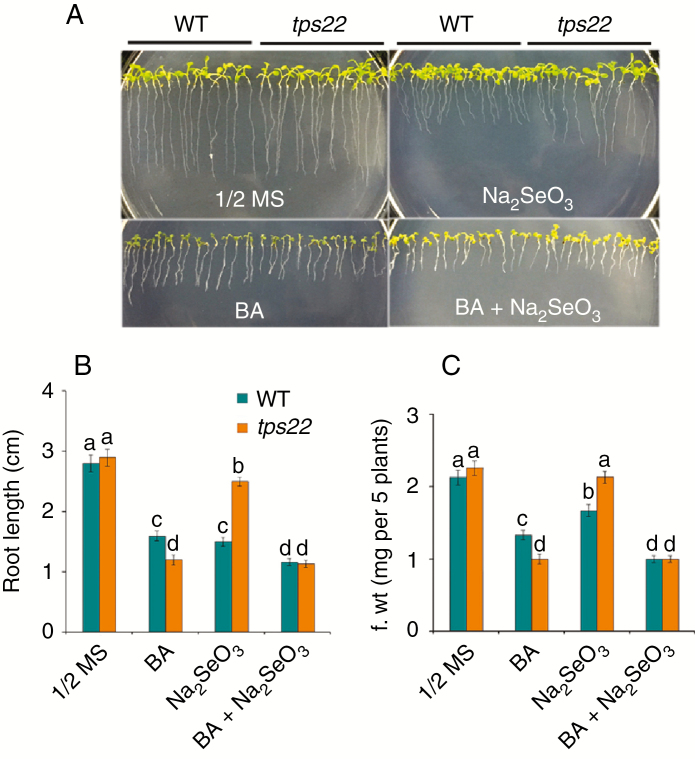
Next, we tested whether application of exogenous CK had any effects on TPS22-mediated Se tolerance. WT and tps22 seedlings grown on ½ MS media with or without BA were challenged by Se stress. In BA-containing medium, root growth was inhibited more severely in tps22 than in WT seedlings, and upon Se challenge, the root length of tps22 plants was less reduced than that of WT plants. When BA was added together with Se, root growth was inhibited severely in both WT and tps22 seedlings and Se tolerance was abolished in tps22 seedlings (Fig. 7). In addition, we also measured the growth of ipt1 3 5 7 mutants (Miyawaki et al., 2006) under Se stress. The ipt1 3 5 7 plants, carrying a quadruple mutation in the key enzyme of CK biosynthesis, had longer roots than the WT on ½ MS media, and showed greater resistance than WT in the presence of Na2SeO3 (Fig. S4). These results suggest that CK plays a negative role in the response to Se stress.
Fig. 7.
Cytokinin (BA) treatment reduced Se tolerance. (A) Analysis of Se tolerance in WT and tps22 seedlings in the absence or presence of Se and BA. WT and tps22 lines were grown on ½ MS media with or without 30 μm Na2SeO3 in the absence or presence of 0.01 μm BA for 12 d. (B,C) Root length (B) and fresh weight (C) of the plants described in A. Data are the mean ± s.e. of three independent replicates. Significant differences (P < 0.05) are indicated by different lowercase letters.
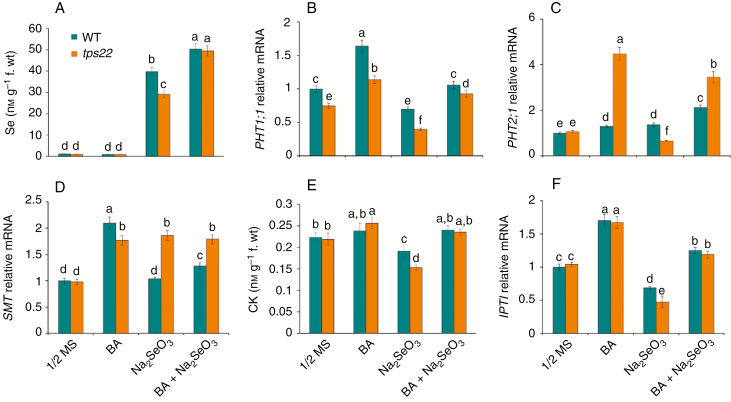
To understand the mechanism underlying CK involved in the regulation of Se tolerance, we determined the effects of BA on Se accumulation under Se stress. BA application significantly enhanced Se accumulation in both WT and tps22 plants and no obvious differences between the WT and tps22 plants grown on ½ MS media containing Na2SeO3 (Fig. 8A). We also examined the effects of BA on the key factors of Se uptake and metabolism detoxification. As shown in Fig. 8B–D, BA significantly enhanced PHT1;1, PHT2;1 and SMT transcription in tps22 seedlings grown on ½ MS media with or without Na2SeO3, suggesting that enhanced Se accumulation in tps22 plants was associated with activation of the transporters, especially PHT2;1. In addition, endogenous CK content and IPT1 transcription were correspondingly increased in both WT and tps22 plants with BA treatment (Fig. 8E, F). TPS25 is the related protein of TPS22 and both enzymes are sesquiterpene synthases that appear to be localized to mitochondria (Tholl and Lee, 2011). TPS25 transcription increased more in tps22 than in WT plants under BA treatment (Fig. S5), which might affect endogenous CK level (Fig. 8E) and result in tps22 seedlings being more sensitive to BA than the WT (Fig. 7).
Fig. 8.
.Effects of exogenous cytokinin (BA) on Se uptake and assimilation. (A) Se contents in WT and tps22 seedlings in the absence or presence of Se and BA. (B–D) qRT-PCR analysis of genes involved in Se uptake and assimilation, including PHT1;1 (B), PHT2;1 (C) and SMT (D). (E) CK contents in WT and tps22 seedlings in the absence or presence of Se and BA. (F) qRT-PCR analysis of the IPT1 gene. WT and tps22 lines were grown on ½ MS media with or without 30 μm Na2SeO3 in the absence or presence of 0.01 μm BA for 12 d, and seedlings were sampled to analyse the index. Data are the mean ± s.e. of three independent replicates. Significant differences (P < 0.05) between parameters are indicated by different lowercase letters.
Together, the above results suggest that CK, through affecting the key factors of Se uptake and metabolism detoxification, regulates TPS22-mediated Se tolerance.
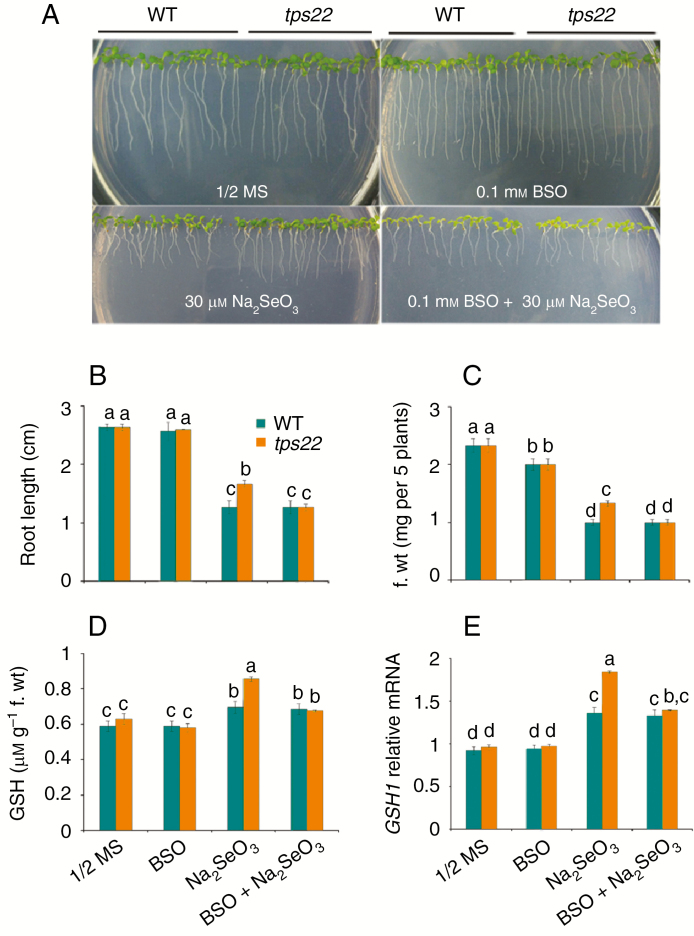
GSH is required for TPS22-mediated Se tolerance
Reduction of selenite in plants might be mediated by glutathione (Van Hoewyk, 2013). To test whether the TPS22-mediated Se resistance was related to GSH, we compared the growth of the tps22 mutant and WT plants in media containing BSO, an inhibitor of GSH synthesis. In ½ MS or 0.1 mm BSO-containing medium, growth of tps22 and WT plants was similar. In the 30 μm-Na2SeO3-containing medium, tps22 plants showed higher root length and fresh weight compared to the WT, but when BSO was added the growth of tps22 plants was similar to that of the WT and the tolerance to Na2SeO3 disappeared (Fig. 9A–C). Moreover, the tps22 seeedlings showed a higher increased GSH content and a higher transcript level of GSH1, the key factor controlling GSH synthesis (Noctor et al., 2012), relative to WT plants (Fig. 9D, E).These results indicate that GSH is required for TPS22-mediated Se tolerance.
Fig. 9.
GSH is required for TPS22-mediated Se tolerance. (A) Effects of buthionine sulfoximine (BSO) on the growth of WT and tps22 plants. WT and tps22 lines were grown on ½ MS media in the absence or presence of 0.1 mm BSO or 30 μm Na2SeO3 for 10 d. (B,C) Root length (B) and fresh weight (C) of the plants described in A. (D,E) GSH contents (D) and GSH1 transcripts (E) in WT and tps22 seedlings in the absence or presence of 0.1 mm BSO or 30 μm Na2SeO3 for 10 d. Data are the mean ± s.e. of three independent replicates. Significant differences (P < 0.05) are indicated by different lowercase letters.
DISCUSSION
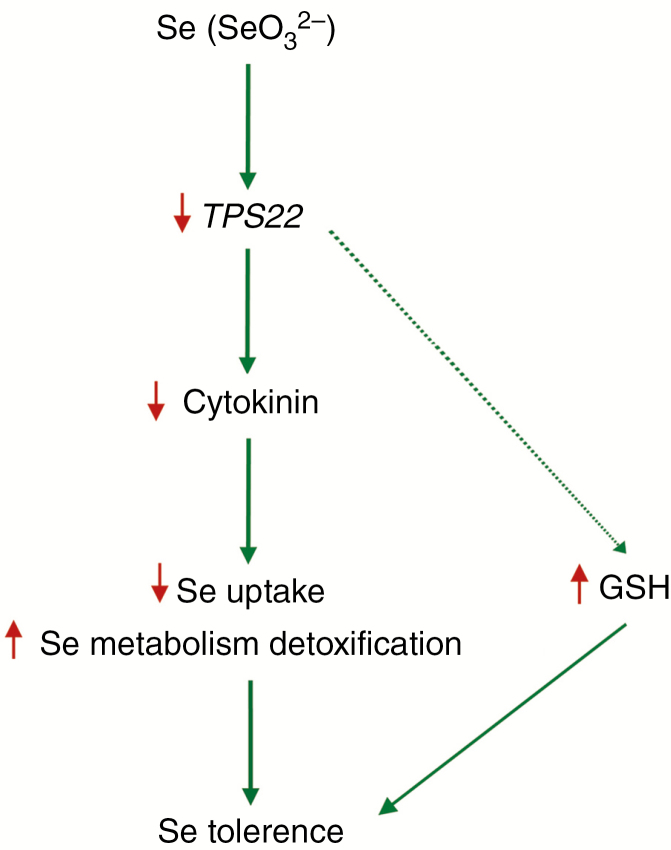
The Arabidopsis TPS superfamily of 40 terpenoid synthase genes consists of seven different subfamilies. AtTPS22 protein is in the TPS-a family (Aubourg et al., 2002; Tholl and Lee, 2011). In this study, we explored the role of TPS22 in Se tolerance in Arabidopsis based on genetic evidence of loss-of-function of TPS22 plant responses to Se stress. Our results demonstrated that TPS22 mediated Se tolerance through the CK signalling pathway. Se stress downregulated TPS22, which then reduced endogenous CK level. Reduced CK, acting through the two-component signalling pathway, affected the key factors of Se uptake and metabolism detoxification, and led to reduction of Se uptake and activation of metabolism detoxification. In addition, increased GSH alleviated the toxicity of excess Se. This cascade of events resulted in reduced Se accumulation and enhanced Se tolerance (Fig. 10).
Fig. 10.
A working model for TPS22-mediated Se tolerance. Se stress downregulated TPS22 expression, which reduced endogenous cytokinin level. Reduced cytokinin, acting through the two-component signalling pathway, decreased Se uptake and increased Se metabolism detoxification ability by affecting expression of the key genes. In addition, increased GSH alleviated the toxicity of excess Se. These events resulted in reduced Se accumulation and enhanced Se tolerance. Downward red arrows indicate decrease, while upward red arrows indicate increase.
Se is an essential micronutrient for many life forms yet is toxic to organisms at high tissue levels (Pilon-Smits et al., 2017). In plants, selenite uptake and Se accumulation were correlated with phosphate transporters (Li et al., 2008; Zhang et al., 2014; Schiavon and Pilon-Smits, 2017). Here, the lower expression level of Pi transporters contributed to decreased Se content and enhanced Se resistance in tps22 mutants under Se stress (Fig. 3). Relative to other micronutrients, the window between Se adequacy and Se toxicity is very narrow. Se toxicity, for plants, is both due to oxidative stress and because Se-amino acids are non-specifically incorporated into proteins, resulting in protein inactivation or denaturation (Pilon-Smits et al., 2017). Therefore, plants have evolved different strategies to cope with Se toxicity. One of the most important mechanisms is the conversion of Se-amino acids into less harmful volatile compounds. SMT has been proposed to play a crucial role in Se detoxification given its high efficiency in methylation of selenocysteine to convert Se to less toxic forms (Lyi et al., 2005; Cakir and Ari, 2013). Higher expression of the SMT gene led to a significant increase in Se tolerance, accumulation and volatilization in both Arabidopsis and Indian mustard (LeDuc et al., 2004, 2006). BoSMT (Broccoli SMT) expression is upregulated by selenate treatment (Lyi et al., 2005). In this study, we detected ignificantly higher transcription levels of SMT in tps22 plants in response to Se stress (Fig. 4), which contributed to enhanced Se resistance in the tps22 mutant.
Phytohormones play an important role in Se resistance. Ethylene, jasmonic acid and salicylic acid signal act to induce Se uptake and Se metabolic and stress-related genes (Freeman et al., 2010; Tamaoki et al., 2008a; Pilon-Smits et al., 2017). Recent research has shown that CK is involved in the regulation of abiotic stress responses through a complex network of CK signalling (Argueso et al., 2009; Ha et al., 2012; Nishiyama et al., 2012; Jeon & Kim, 2013; Zwack et al., 2013; Li et al., 2016). It is generally considered that CKs play a negative role in plant adaptation to stress by a reduction of active CK levels or by repression of CK signalling components (Ha et al., 2012; Kieber and Schaller, 2014; Zwack and Rashotte, 2015). Thus, the reduced level of CK in the tps22 mutant led to increased root length and tolerance compared with the WT subjected to Se. The lower AHKs and significantly higher ARR3, ARR15 and ARR16 transcript levels in the tps22 mutant suggest that enhanced Se tolerance in tps22 is due to repression of AHKs and negative feedback of the type-A ARR3, ARR15 and ARR16 (Figs 1 and 6). CK treatment increased sodium accumulation in Arabidopsis seedlings (Mason et al., 2010). The expression of the high-affinity potassium transporter AtHKT1.1, which removes sodium from the root, was repressed by CK treatment (Mason et al., 2010). In contrast, our results showed that transcriptions of phosphate transporters PHT1;1 and PHT2;1 were upregulated by CK treatment (Fig. 8), which increased Se accumulation, and as a result, the resistance of the tps22 mutant to Se was completely altered and disappeared.
Selenite treatment induces oxidative stress. GSH is associated with improved tolerance to oxidative stress and Se stress in plants (Noctor et al., 2012; Jiang et al., 2016). GSH-mediated reduction of selenite, the reduction of selenite to selenide, may be an interaction between selenite and reduced GSH (Schiavon and Pilon-Smits, 2017). Our BSO test indicated that TPS22-mediated Se tolerance also occurs through the GSH detoxification pathway, by which the expression level of GSH1 was improved and the content of GSH was increased to alleviate Se toxicity. In addition, GPX1 transcript and GPX activity in tps22 plants increased more to cope with ROS compared to those in the WT, and therefore we observed lower ROS accumulation in the mutant under Se stress (Figs 4 and 5).
In summary, our results suggest that loss-of-function of TPS22 resulted in decreased Se accumulation and enhanced Se tolerance in Arabidopsis. CK is involved in TPS22-mediated Se tolerance by affecting expression of the key genes of Se uptake and Se metabolism detoxification.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: Primer sequences used for RT-PCR and qRT-PCR. Fig. S1: Growth of wild-type (WT) and tps22 plants in soil-filled pots. Fig. S2: Phenotypes of WT, tps22 and their F1 plants grown on ½ MS media in the absence or presence of 30 μm Na2SeO3 for 3 weeks. Fig. S3: Pi contents in WT and tps22 plants grown on ½ MS media with or without 30 μm Na2SeO3 for 2 weeks. Fig. S4: The phenotypes of WT and ipt1 3 5 7 plants grown on ½ MS media with or without 30 μm Na2SeO3 for 2 weeks. Fig. S5: TPS25 transcript level determined by qRT-PCR analysis.
ACKNOWLEDGMENTS
We thank Dr Dongwei Di (Institute of Soil Science, Chinese Academy of Sciences, Nanjing) who kindly provided seeds of the ipt1 3 5 7 mutant. This work was supported by the Major Projects of Science and Technology in Anhui Province (17030701024), the Key Research and Development Project of Anhui Province (1704g07020110) and Anhui Provincial Natural Science Foundation (1208085MC47).
LITERATURE CITED
- Ames BN. 1966. Assay of inorganic phosphate, total phosphate and phosphatases. Methods in Enzymology 8: 115–118. [Google Scholar]
- Agalou A, Roussis A, Spaink HP. 2005. The Arabidopsis selenium-binding protein confers tolerance to toxic levels of selenium. Functional Plant Biology 32: 881–890. [DOI] [PubMed] [Google Scholar]
- Argueso CT, Ferreira FJ, Kieber JJ. 2009. Environmental perception avenues: the interaction of cytokinin and environmental response pathways. Plant Cell and Environment 32: 1147–1160. [DOI] [PubMed] [Google Scholar]
- Aubourg S, Lecharny A, Bohlmann J. 2002. Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Molecular Genetics and Genomics 267: 730–745. [DOI] [PubMed] [Google Scholar]
- Banuelos GS, Fakra SC, Walse SS. 2011. Selenium accumulation, distribution, and speciation in spineless prickly pear cactus: A drought- and salt-tolerant, selenium-enriched nutraceutical fruit crop for biofortified foods. Plant Physiology 155: 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabannes E, Buchner P, Broadley MR. 2011. A comparison of sulfate and selenium accumulation in relation to the expression of sulfate transporter genes in Astragalus species. Plant Physiology 157: 2227–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir O, Ari S. 2013. Cloning and molecular characterization of selenocysteine methyltransferase (AchSMT) cDNA from Astragalus chrysochlorus. Plant Omics Journal 6: 100–106. [Google Scholar]
- Chen F, Tholl D, Bohlmann J, Pichersky E. 2011The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. The Plant Journal 66: 212–229. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Daram P, Brunner S, Rausch C, Steiner C, Amrhein N, Bucher M. 1999. Pht2;1 encodes a low-affinity phosphate transporter from Arabidopsis. Plant Cell 11: 2153–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R, Wei C, Tu S. 2013. The roles of selenium in protecting plants against abiotic stresses. Environmental and Experimental Botany 87: 58–68. [Google Scholar]
- Freeman JL, Tamaoki M, Stushnoff C. 2010. Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiology 153: 1630–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falara V, Akhtar TA, Nguyen TT, et al. . 2011. The tomato terpene synthase gene family. Plant Physiology 157: 770–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel-Jensen C, Gammelgaard B. 2010. Selenium metabolism in hepatocytes incubated with selenite, selenate, selenomethionine, Se-methylselenocysteine and methylseleninc acid and analysed by LC-ICP-MS. Journal of Analytical Atomic Spectrometry 25: 414–418. [Google Scholar]
- Gupta M, Gupta S. 2017. An overview of selenium uptake, metabolism, and toxicity in plants. Frontiers in Plant Science 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Tran LP. 2012. Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends in Plant Science 17: 172–179. [DOI] [PubMed] [Google Scholar]
- Huang QQ, Wang Q, Luo Z, Yu Y, Jiang RF, Li HF. 2015. Effects of root iron plaque on selenite and selenate dynamics in rhizosphere and uptake by rice (Oryza sativa). Plant and Soil 388: 255–266. [Google Scholar]
- Hugouvieux V, Dutilleul C, Jourdain A, Reynaud F, Lopez V, Bourguignon J. 2009. Arabidopsis putative selenium-binding protein 1 expression is tightly linked to cellular sulfur demand and can reduce sensitivity to stresses requiring glutathione for tolerance. Plant Physiology 151: 768–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Kim J. 2013. Arabidopsis response Regulator1 and Arabidopsis histidine phosphotransfer Protein2 (AHP2), AHP3, and AHP5 function in cold signaling. Plant Physiology 161: 408–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Chen ZP, Gao QC, et al. . 2016. Loss-of-function mutations in the APX1 gene result in enhanced selenium tolerance in Arabidopsis thaliana. Plant Cell and Environment 39: 2133–2144. [DOI] [PubMed] [Google Scholar]
- Jiang L, Wang WY, Chen ZP, Gao QC, Xu QX, Cao HM. 2017. A role for APX1 gene in lead tolerance in Arabidopsis thaliana. Plant Science 256: 94–102. [DOI] [PubMed] [Google Scholar]
- Kamada-Nobusada T, Sakakibara H. 2009. Molecular basis for cytokinin biosynthesis. Phytochemistry 70: 444–449. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Schaller GE. 2014. Cytokinins. The Arabidopsis Book 12: e0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDuc DL, Tarun AS, Montes-Bayon M, et al. . 2004. Overexpression of selenocysteine methyltransferase in Arabidopsis and Indian mustard increases selenium tolerance and accumulation. Plant Physiology 135: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDuc DL, AbdelSamie M, Móntes-Bayon M, Wu CP, Reisinger SJ, Terry N. 2006. Overexpressing both ATP sulfurylase and selenocysteine methyltransferase enhances selenium phytoremediation traits in Indian mustard. Environmental Pollution 144: 70–76. [DOI] [PubMed] [Google Scholar]
- Lehotai N, Petô A, Erdei L, Kolbert Z. 2011. The effect of selenium (Se) on development and nitric oxide levels in Arabidopsis thaliana seedlings. Acta Biologica Szegediensis 55: 105–107. [Google Scholar]
- Lehotai N, Kolbert Z, Pető A, et al. . 2012. Selenite-induced hormonal and signaling mechanisms during root growth of Arabidopsis thaliana L. Journal of Experimental Botany 63: 5677–5687. [DOI] [PubMed] [Google Scholar]
- Li HF, McGrath SP, Zhao FJ. 2008. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytologist 178: 92–102. [DOI] [PubMed] [Google Scholar]
- Li W, Herrera-Estrella L, Tran LP. 2016. The Yin–Yang of cytokinin homeostasis and drought acclimation/ adaptation. Trends in Plant Science 21: 548–550. [DOI] [PubMed] [Google Scholar]
- Liu XW, Zhao ZQ, Hu CX, Zhao XH, Guo ZH. 2016. Effect of sulphate on selenium uptake and translocation in rape (Brassica napus L.) supplied with selenate or selenite. Plant and Soil 399: 295–304. [Google Scholar]
- Liu YG, Mitsukawa N, Oosumi T, Whittier RF. 1995. Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. The Plant Journal 8: 457–463. [DOI] [PubMed] [Google Scholar]
- Lyi SM, Heller LI, Rutzke M, Welch RM, Kochian LV, Li L. 2005. Molecular and biochemical characterization of the selenocysteine Se-Methyltransferase gene and Se-Methylselenocysteine synthesis in Broccoli. Plant Physiology 138: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Jha D, Hill K, et al. . 2010. Cytokinin regulates sodium accumulation in the shoots of Arabidopsis thaliana. Plant Journal 64: 753–763. [DOI] [PubMed] [Google Scholar]
- McConnell KP, Portman OW. 1952. Toxicity of dimethyl selenide in the rat and mouse. Experimental Biology and Medicine 79: 230–231. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, et al. . 2006. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proceedings of the National Academy of Sciences USA 103: 16598–16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Sheen J. 2007. Advances in cytokinin signaling. Science 318: 68–69. [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473–497. [Google Scholar]
- Nishiyama R, Le DT, Watanabe Y, et al. . 2012. Transcriptome analyses of a salt-tolerant cytokinin-deficient mutant reveal differential regulation of salt stress response by cytokinin deficiency. PLoS One 7: e32124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. 1998. Simultaneous measurement of foliar glutathione, c-glutamylcysteine, and amino acids by high-performance liquid chromatography: comparison with the two other assay methods for glutathione. Analytical Biochemistry 264: 98–110. [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Chaouch S, et al. . 2012Glutathione in plants: an integrated overview. Plant Cell and Environment 35: 454–484. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits EAH. 2015. Selenium in plants. Progress in Botany 76: 93–108. [Google Scholar]
- Pilon-Smits EAH, Le Duc D. 2009. Phytoremediation of selenium using transgenic plants. Current Opinion in Biotechnology 20: 207–212. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Hwang S, Mel LC, et al. . 1999. Overexpression of ATP sulfurylase in indian mustard leads to increased selenate uptake, reduction, and tolerance. Focus on Autism & Other Developmental Disabilities 119: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Garifullina GF, Abdel-Ghany SE, et al. . 2002. Characterization of a NifS-like chloroplast protein from Arabidopsis. Implications for its role in sulfur and selenium metabolism. Plant Physiology 130: 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Winkel LHE, Lin ZQ. 2017. Selenium in plants. Plant Ecophysiology 11: 3–66. [Google Scholar]
- Remy E, Cabrito TR, Batista RA, Teixeira MC, Sá-Correia I, Duque P. 2012. The Pht1;9 and Pht1;8 transporters mediate inorganic phosphate acquisition by the Arabidopsis thaliana root during phosphorus starvation. New Phytologist 195: 356–371. [DOI] [PubMed] [Google Scholar]
- Ro DK, Ehlting J, Keeling CI, Lin R, Mattheus N, Bohlmann J. 2006. Microarray expression profiling and functional characterization of AtTPS genes: duplicated Arabidopsis thaliana sesquiterpene synthase genes At4g13280 and At4g13300 encode root-specific and wound-inducible (Z)-gamma-bisabolene synthases. Archives of Biochemistry and Biophysics 448: 104–116. [DOI] [PubMed] [Google Scholar]
- Schiavon M, Pilon-Smits EAH. 2017. The fascinating facets of plant selenium accumulation – biochemistry, physiology, evolution and ecology. New Phytologist 213: 1582–1596. [DOI] [PubMed] [Google Scholar]
- Sors TG, Ellis DR, Salt DE. 2005. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynthesis Research 86: 373–389. [DOI] [PubMed] [Google Scholar]
- Stadtman TC. 1990. Selenium biochemistry. Annual Review of Biochemistry 59: 111–127. [DOI] [PubMed] [Google Scholar]
- Sun JQ, Niu QW, Tarkowski P, et al. . 2003. The Arabidopsis AtIPT8/PGA22 gene encodes an isopentenyltransferase that is involved in de novo cytokinin biosynthesis. Plant Physiology 131: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki M, Freeman JL, Marquès L, Pilon-Smits EA. 2008a. New insights into the roles of ethylene and jasmonic acidin the acquisition of selenium resistance in plants. Plant Signaling and Behavior 3: 865–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki M, Freeman JL, Pilon-Smits EA. 2008b. Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis. Plant Physiology 146: 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappel AL. 1978. Glutathione peroxidase and hydroperoxidas. Methods in Enzymology 77: 735–740. [DOI] [PubMed] [Google Scholar]
- Terry N, Zayed AM, De Souza MP, Tarun AS. 2000. Selenium in higher plants. Annual Review of Plant Biology 51: 401–432. [DOI] [PubMed] [Google Scholar]
- Tholl D, Lee S. 2011. Terpene specialized metabolism in Arabidopsis thaliana. Arabidopsis Book 9: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoewyk D. 2013. A tale of two toxicities: malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Annals of Botany 112: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoewyk D, Garifullina GF, Ackley AR, et al. . 2005. Overexpression of AtCpNifS enhances selenium tolerance and accumulation in Arabidopsis. Plant Physiology 139: 1518–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versaw WK, Harrison MJ. 2002. A chloroplast phosphate transporter, pht2;1, influences allocation of phosphate within the plant and phosphate-starvation responses. Plant Cell 14: 1751–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber CG. 1980. Toxicology of selenium: a review. Clinical Toxicology 17: 171–230. [DOI] [PubMed] [Google Scholar]
- Xie YJ, Xu S, Han B, et al. . 2011. Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. The Plant Journal 66: 280–292. [DOI] [PubMed] [Google Scholar]
- Ye H, Garifullina GF, Abdel-Ghany SE, Lihong Z, Pilon-Smits EAH, Pilon M. 2005. The chloroplast NifS-like protein of Arabidopsis thaliana is required for iron- sulfur cluster formation in ferredoxin. Planta 220: 602–608. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN. 2006. Statistical analysis of real-time PCR data. BMC Bioinformatics 7: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xu JX, Kong YZ, et al. . 2005. Generation of chemical-inducible activation tagging T-DNA insertion lines of Arabidopsis thaliana. Acta Genetica Sinica 32: 1082–1088. [PubMed] [Google Scholar]
- Zhang LH, Hu B, Li W, et al. . 2014. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytologist 201: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YG, Pilon-Smits EA, Zhao FJ, Williams PN, Meharg AA. 2009. Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends in Plant Science 14: 436–442. [DOI] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Chua NH. 2000. An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant Journal 24: 265–273. [DOI] [PubMed] [Google Scholar]
- Zwack PJ, Rashotte AM. 2015. Interactions between cytokinin signalling and abiotic stress responses. Journal of Experimental Botany 66: 4863–4871. [DOI] [PubMed] [Google Scholar]
- Zwack PJ, Robinson BR, Risley MG, Rashotte AM. 2013. Cytokinin Response Factor 6 negatively regulates leaf senescence and is induced in response to cytokinin and numerous abiotic stresses. Plant Cell Physiology 54: 971–981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.