Abstract
SMYD4 belongs to a family of lysine methyltransferases. We analyzed the role of smyd4 in zebrafish development by generating a smyd4 mutant zebrafish line (smyd4L544Efs*1) using the CRISPR/Cas9 technology. The maternal and zygotic smyd4L544Efs*1 mutants demonstrated severe cardiac malformations, including defects in left-right patterning and looping and hypoplastic ventricles, suggesting that smyd4 was critical for heart development. Importantly, we identified two rare SMYD4 genetic variants in a 208-patient cohort with congenital heart defects. Both biochemical and functional analyses indicated that SMYD4(G345D) was pathogenic. Our data suggested that smyd4 functions as a histone methyltransferase and, by interacting with HDAC1, also serves as a potential modulator for histone acetylation. Transcriptome and bioinformatics analyses of smyd4L544Efs*1 and wild-type developing hearts suggested that smyd4 is a key epigenetic regulator involved in regulating endoplasmic reticulum-mediated protein processing and several important metabolic pathways in developing zebrafish hearts.
Author summary
SMYD4 belongs to a SET and MYND domain-containing lysine methyltransferase. In zebrafish, smyd4 is ubiquitously expressed in early embryos and becomes enriched in the developing heart at 48 hours post-fertilization (hpf). We generated a smyd4 mutant zebrafish line (smyd4L544Efs*1) using the CRISPR/Cas9 technology. The maternal and zygotic smyd4L544Efs*1 mutants demonstrated a strong defect in cardiomyocyte proliferation, which led to a severe cardiac malformation, including left-right looping defects and hypoplastic ventricles. More importantly, two rare genetic variants of SMYD4 were enriched in a 208-patient cohort with congenital heart defects. Both biochemical and functional analyses indicated that SMYD4(G345D) was highly pathogenic. Using mass spectrometric analysis, SMYD4 was shown to specifically interact with histone deacetylase 1 (HDAC1) via its MYND domain. Altered di- and tri-methylation of histone 3 lysine 4 (H3K4me2 and H3K4me3) and acetylation of histone 3 in smyd4L544Efs*1 mutants suggested that smyd4 plays an important role in epigenetic regulation. Transcriptome and pathway analyses demonstrated that the expression levels of 3,856 genes were significantly altered, which included cardiac contractile genes, key signaling pathways in cardiac development, the endoplasmic reticulum-mediated protein processing pathway, and several important metabolic pathways. Taken together, our data suggests that smyd4 is a key epigenetic regulator of cardiac development.
Introduction
Protein post-translational modifications (PTMs) are critical for the biological function of proteins. Histone modification is a common epigenetic mechanism that plays essential roles in the regulation of chromatin structure and gene expression. Different types of histone modifications, which are mediated by a series of specific enzymes, can either enhance or inhibit transcription to regulate specific cellular functions or signaling pathways. SET and MYND domain-containing proteins (SMYDs) belong to a unique family of histone lysine methyltransferases. This family is composed of five members, including SMYD1, SMYD2, SMYD3, SMDY4, and SMYD5. These proteins share a Su(var)3-9, an Enhancer-of-zeste and Trithorax (SET) domain with lysine-specific methyltransferase activity, a Myeloid, Nervy, and DEAF-1 (MYND) domain, and a tetratricopeptide repeat (TPR) domain, which are involved in protein-protein interactions [1–3]. Several biochemical studies and functional analyses showed that SMYDs 1–3 exhibit methyltransferase activities for both histone and non-histone proteins [3–6]. SMYD members are widely present in multiple cell types, including those of skeletal and cardiac muscles [7–9]. Genetic ablation of Smyd1 in mice led to defects in right ventricular development [8,10]. The knockdown of smyd1, smyd2, and smyd3 in zebrafish using morpholino technology also led to cardiac malformation and defects in skeletal and cardiac myofibrillogenesis [11–13]. Zebrafish smyd5 was recently reported to play important roles in hematopoiesis [14]. Our knowledge of the biological function of SMYD4 remains limited. A previous study found that dSMYD4 was crucial for muscle development in Drosophila [15]. However, the role of SMYD4 in vertebrate development and its underlying molecular mechanism have not yet been analyzed thoroughly.
Congenital heart diseases (CHDs) are the most common birth defects, with an annual incidence of approximately 1% of newborns worldwide [16]. Heart development involves complex genetic and epigenetic regulation [17–19]. Cardiac-specific ablation of HDAC1 and HDAC2 in the murine heart led to aberrant gene expression, contributing to defects in cardiac morphogenesis and contractility [20], suggesting the critical roles of histone modification and epigenetic regulation in cardiac development. The significant enrichment of mutations in genes encoding chromatin modifiers in patients with CHDs provided further evidence to support this notion [21,22].
In this study, we analyzed smyd4 expression in zebrafish embryos and generated a smyd4 loss-of-function mutation (smyd4L544Efs*1) using the CRISPR/Cas9 genome editing technology. We demonstrated that smyd4 is critical for cardiac malformation in zebrafish development. Two rare missense SMYD4 variants were identified in patients with CHDs. Both in vitro biochemical assays and in vivo functional analyses strongly suggested that the variant SMYD4(G345D) was pathogenic. Our data suggested that smyd4 functions as a histone methyltransferase and, by interacting with HDAC1, also serves as a potential modulator for histone acetylation. Transcriptome and bioinformatics analyses of the differentially expressed genes in developing hearts isolated from maternal and zygotic smyd4L544Efs*1 (MZsmyd4L544Efs*1) mutant and normal control embryos showed a significant enrichment of the key genes involved in cardiac development and contractile function, and the endoplasmic reticulum-mediated protein processing pathway and several important metabolic pathways. Taken together, our results suggest that smyd4, in association with hdac1, is an important epigenetic regulator of these critical pathways during zebrafish cardiac development.
Results
smyd4 expression is specifically enriched in the developing heart
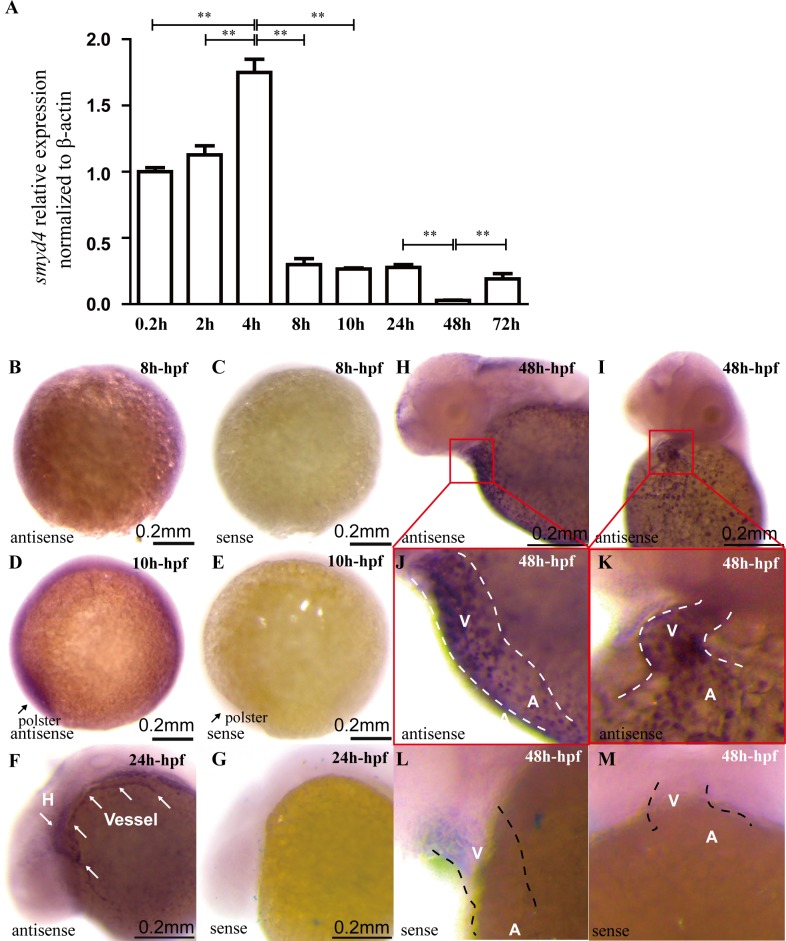
To determine the smyd4 mRNA levels in early to late zebrafish embryos, we performed a qRT-PCR analysis on embryos at the single-cell (0.2 hours post-fertilization (hpf)), 64-cell (2 hpf), blastula (4 hpf), mid-gastrula (8 hpf), late-gastrula (10 hpf), early primordium (24 hpf), long pectoral (48 hpf), and protruding mouth stage (72 hpf) stages (Fig 1A). We found that smyd4 transcripts were highly present in the single-cell stage, suggesting that a large amount of smyd4 was present as maternal transcripts. Zygotic smyd4 transcription peaked at the blastula stage and was followed by downregulation in the gastrula stage, indicating the role of this transcript in zebrafish early development. The overall expression levels of smyd4 were significantly further downregulated at 48 hpf and followed by a quick reactivation of expression at 72 hpf. We then used whole-mount in situ hybridization to determine the spatio-temporal expression pattern of smyd4 in developing zebrafish embryos. As shown in Fig 1B–1E, smyd4 was expressed ubiquitously in mid- and late-gastrula embryos; the highest level of expression was found at the polster in late-gastrula embryos (Fig 1D and 1E). By 24 hpf, smyd4 transcripts were found to be enriched in the developing heart and blood vessel (Fig 1F and 1G) and became more restricted to the cardiovascular system at 48 hpf (Fig 1H–1M). Interestingly, the developing ventricle had a significantly higher level of smyd4 expression than the atrium (Fig 1J and 1K), suggesting its role in cardiac development. The reactivation of smyd4 expression at 72 hpf appeared not to be cardiac specific (Fig 2F).
Fig 1. Quantitative RT-PCR and whole-mount in situ hybridization and qRT-PCR analyses of smyd4 expression in zebrafish early embryogenesis.
(A) The overall expression levels of smyd4 at 0.2, 2, 4, 8, 10, 24, 48 and 72 hpf were analyzed using qRT-PCR. smyd4 displayed its highest expression levels during the blastula stage (4 hpf) and was significantly decreased at 48 hpf. At 72 hpf, there was a quick reactivation of smyd4 expression. (B-C) During the mid-gastrula stage (8 hpf), smyd4 was ubiquitously expressed in the mid-gastrula embryo (8 hpf). (D, E) smyd4 was expressed ubiquitously at 10 hpf, with the highest expression levels found at the polster. (F, G) smyd4 was abundant in the heart and blood vessel at 24 hpf. (H-M) Lateral and frontal view of smyd4 expression at 48 hpf. smyd4 became more enriched in the developing hearts at 48 hpf, and the ventricle had a higher expression level than that in the atrium. A smyd4 sense probe was used as the negative control for the in situ hybridization analysis.
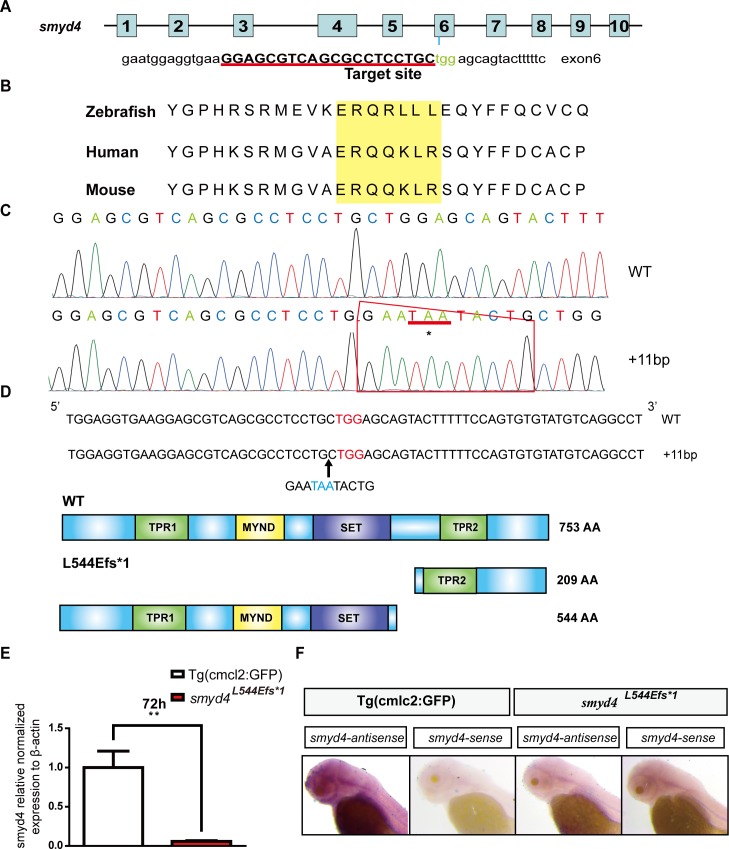
Fig 2. The generation of smyd4–deficient zebrafish using the CRISPR/Cas9 technology.
(A) Schematic diagram of the smyd4 gene structure and the sgRNA3 sequence for the exon 6 targeting site. The sgRNAs were injected into the embryos with Cas9 mRNA at the single-cell stage; (B) The targeting site of sgRNA3 was well-conserved from zebrafish to humans; (C) Sanger sequencing confirmed the insertion mutation (c.1629_1630inGAATAATACTG; p.Leu544Glufs*1); (D) A schematic diagram of the insertion mutation. The smyd4 protein contains four functional domains: two TPR domains (green), one MYND domain (yellow), and one SET domain (purple). The insertion mutation created a truncated mutant protein that lacks the entire C-terminus, which contained the key functional domain TPR2; (E) qRT-PCR confirmation that smyd4 expression is significantly decreased in MZsmyd4L544Efs*1 embryos; (F) Whole-mount in situ hybridization analysis confirmed the loss of smyd4 expression in MZsmyd4L544Efs*1 embryos at 72 hpf.
smyd4-deficient zebrafish is generated by using the CRISPR/Cas9 technology
To determine the biological function of smyd4, we generated smyd4-deficient zebrafish using the CRISPR/Cas9 technology. Three sgRNAs were designed to target different exons of smyd4 that contribute to the ZMYND, C-terminal TPR2, and SET domains. We were able to generate an 11-nt insertion in exon 6 that was targeted by sgRNA3 (Fig 2A). As shown in Fig 2B, the sgRNA3 targeted sequence is well conserved from zebrafish to humans. Sequencing analysis confirmed this 11-nt insertion, which led to a frameshift at amino acid 545 (smyd4L544Efs*1) (Fig 2C and 2D and S1 Fig). This frameshift mutation yielded a premature stop codon (TAA), as indicated by the asterisk in Fig 2C, giving rise to a truncated mutant protein that lacks the entire C-terminus containing the TPR2 domain (Fig 2D). qRT-PCR and in situ hybridization analyses demonstrated that the smyd4 mRNA transcript was completely abolished in MZsmyd4L544Efs*1 embryos (Fig 2E and 2F). The lack of the smyd4 mRNA transcript was likely due to the well-known effect of nonsense mutation-mediated degradation (NMD) [23], a process common to frameshift mutations. Thus, we concluded that smyd4L544Efs*1 was a loss-of-function mutation. To better analyze the cardiac defects in MZsmyd4L544Efs*1 mutants, we crossed the smyd4L544Efs*1 line with the cardiomyocyte-specific transgenic reporter line Tg(cmcl2:GFP). The entire work presented here was performed using this bi-genic line (smyd4L544Efs*1;cmcl2:GFP) with Tg(cmcl2:GFP) as a normal control, unless otherwise indicated in the text.
The smyd4 deficiency leads to abnormal cardiac development
Using the heterozygous breeding scheme, we were able to create heterozygous and homozygous smyd4L544Efs*1 mutant and wild-type embryos. First, we analyzed the survival of smyd4L544Efs*1 homozygous mutants and found no early lethality in these mutants, as the total amount of surviving smyd4L544Efs*1 homozygous mutants reached 25% among all three genotypes, which matched the normal Mendelian ratio. These homozygous mutants appeared normal in terms of growth and reproductivity. However, when examining these mutants, we observed a slight increase in embryos with abnormal cardiac situs ambiguus in smyd4L544Efs*1 homozygous (6 out of 14, 43%) and heterozygous (17 out of 48, 35%) mutants compared to the number of wild-type embryos (2 out of 10, 20%) (S2 Fig). As situs ambiguus is highly relevant for defects in early patterning, this finding prompted us to speculate that the reduced but remaining maternal smyd4 transcripts in heterozygous females might contribute to the reduced severity of the resulting phenotype. Therefore, we established a homozygous breeding scheme to eliminate the effect of maternal smyd4 transcripts.
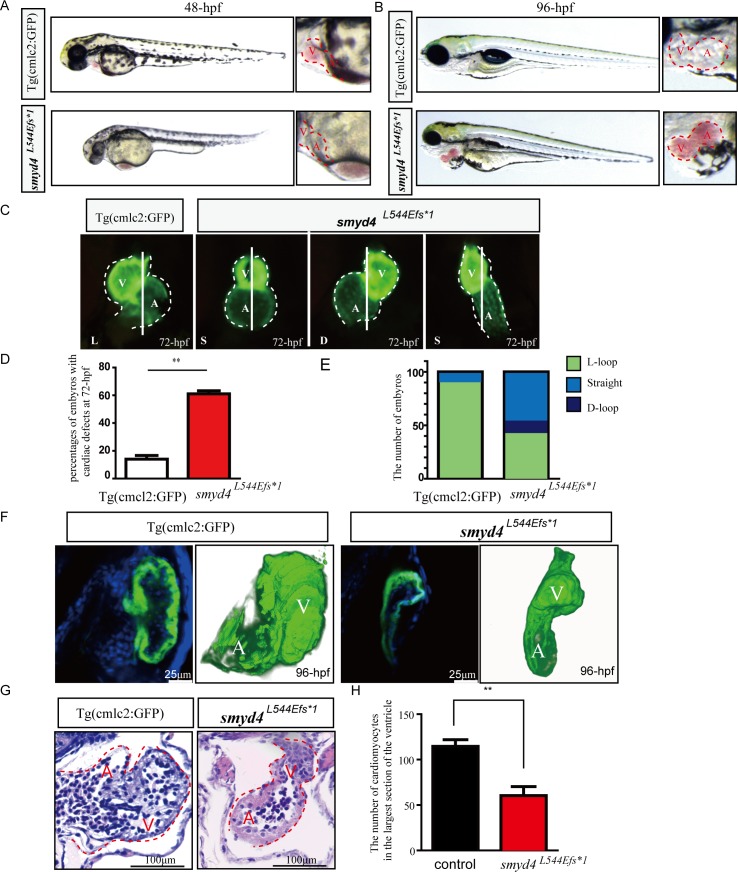
The MZsmyd4L544Efs*1 embryos from the homozygous breeding scheme displayed severe pericardial edema (Fig 3A and 3B) and/or congested blood flow in the ventral veins (Fig 3A and 3B), suggesting prominent cardiac defects or dysfunctions associated with the MZsmyd4L544Efs*1 embryos. The cardiac defects were analyzed carefully. Major defects included a significantly smaller ventricular size and anomalies of cardiac left-right asymmetric patterning or looping in approximately 60% of MZsmyd4L544Efs*1 embryos at 72 hpf (Fig 3C and 3G), such as situs ambiguus (straight heart tube) and situs inversus (D-loop heart tube) (Fig 3C). The 3D-reconstruction of confocal images of 96 hpf hearts further confirmed this looping defect (Fig 3F and S1 and S2 Movies), as well as hypoplastic ventricles with less trabeculated myocardial structures in the MZsmyd4L544Efs*1 hearts (Fig 3F and 3G). The number of cardiomyocytes on the largest section plane of the MZsmyd4L544Efs*1 mutant ventricle was found significantly reduced when compared to the comparable section plane of wild-type control hearts (S3 Fig and Fig 3H), suggesting a great reduction of total number of cardiomyocytes in MZsmyd4L544Efs*1 hearts. To determine whether reduced cell number was due to the decreased cellular proliferative activity, we used p-H3 and PCNA immune reactivities to respective antibodies as the indicators for the level of cell proliferative activities in mutant developing hearts. As shown in Fig 4A and S4 Fig, MZsmyd4L544Efs*1 mutant cardiomyocytes had a dramatically reduced level in cellular proliferation when compared to wild-type controls. In addition, we also evaluated the levels of apoptosis in mutant hearts and we found no evidence of increased level of apoptotic cell in MZsmyd4L544Efs*1 mutant hearts (S5 Fig).
Fig 3. Phenotypical analyses of MZsmyd4L544Efs*1 embryos.
(A) Representative images of the MZsmyd4L544Efs*1 and control embryos at 48 hpf, exhibiting congested blood flow in the ventral vein; (B) Representative images of the MZsmyd4L544Efs*1 and control embryos at 96 hpf, displaying severe pericardial edema; (C) Representative images of the MZsmyd4L544Efs*1 and control hearts at 72 hpf, demonstrating abnormal cardiac morphology and left-right looping defects; (D) Significantly more MZsmyd4L544Efs*1 embryos had abnormal cardiac development than control fish Tg (cmcl2:GFP) (61% ± 4.0% mutants vs. 14% ± 4.6% controls, n = 100, p < 0.01**); (E) Comparison of left-right patterning defects in the MZsmyd4L544Efs*1 (n = 100) and control (n = 100) embryos (green: normal L-loop; blue: straight; dark blue: D-loop). Only 10% of the control embryos showed an abnormal “straight” cardiac left-right asymmetry pattern. However, 48% of MZsmyd4L544Efs*1 embryos exhibited a “straight” left-right asymmetry pattern, and 11% showed a “D-loop” left-right asymmetry pattern; (F) Representative confocal images of the ventricular wall and 3D re-construction images of the MZsmyd4L544Efs*1 and control hearts at 96 hpf. The confocal scanned images demonstrated hypoplastic wall with significant reduction of trabeculation in MZsmyd4L544Efs*1 ventricle when compared to the wild-type control ventricle. The 3D-reconstruction of the hearts confirmed left-right looping defects in MZsmyd4L544Efs*1 hearts (For a better view of 3D-structures, see supplemental material S1 and S2 Movies). (G) Histological sections and H&E staining images of the MZsmyd4L544Efs*1 and control hearts at 96 hpf, demonstrating abnormal ventricular walls and reduced trabeculation; (H) The quantitative analysis of the number of cardiomyocytes in the ventricle displayed that MZsmyd4 L544Efs*1 had significantly reduced cardiomyocytes (p<0.01, **).
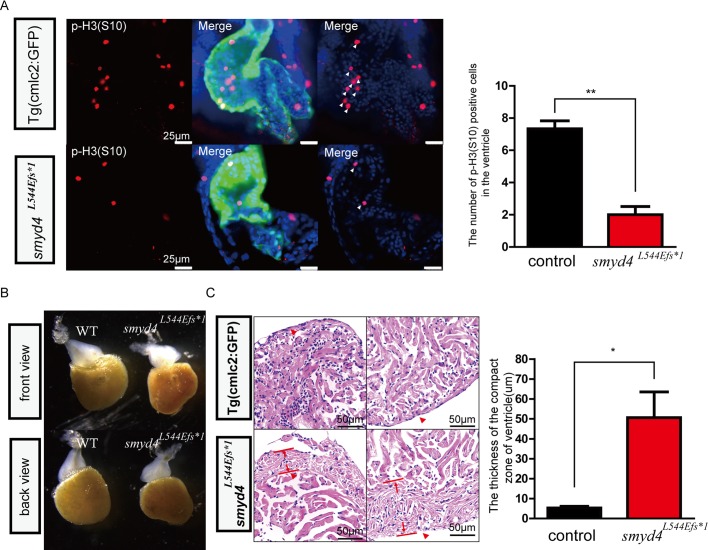
Fig 4. Phenotypical analyses of MZsmyd4L544Efs*1 embryos.
(A) The p-H3(S10) immunofluorescence staining showed decreased cell proliferative activity in MZsmyd4L544Efs*1 cardiomyocytes at 48 hpf. The number of p-H3 (S10) positive cells in the MZsmyd4L544Efs*1 ventricles was significantly decreased when compared to the control group (p<0.01, **); (B) The abnormal ventricular morphology of the adult MZsmyd4L544Efs*1 hearts compared to the morphology of control adult hearts; (C) Histological sections and H&E staining images of the adult MZsmyd4L544Efs*1 and control hearts, demonstrating significant thickening of the compact ventricular wall. (The red arrows showed that in the similar compact zone of the ventricle). The quantitative analysis displayed that the thickness of the ventricle compact zone was significantly increased in adult MZsmyd4 L544Efs*1 hearts compared to Tg(cmcl2:GFP) (p<0.05, *).
As adults, these MZsmyd4L544Efs*1 mutants had abnormal gross cardiac morphologies and histologies, typically with less trabecular myocardia and sometimes with a dramatically increased thickness of the ventricular compact wall (Fig 4B and 4C). Despite these cardiac defects, we observed no apparent defects in skeletal muscle development in MZsmyd4L544Efs*1 mutants examined at 48 and 72 hpf (S6 Fig), suggesting that smyd4 is lesser important than smyd1 in vertebrate muscle development [11].
SMYD4 interacts with HDAC1, and altered histone modification occurs in MZsmyd4L544Efs*1
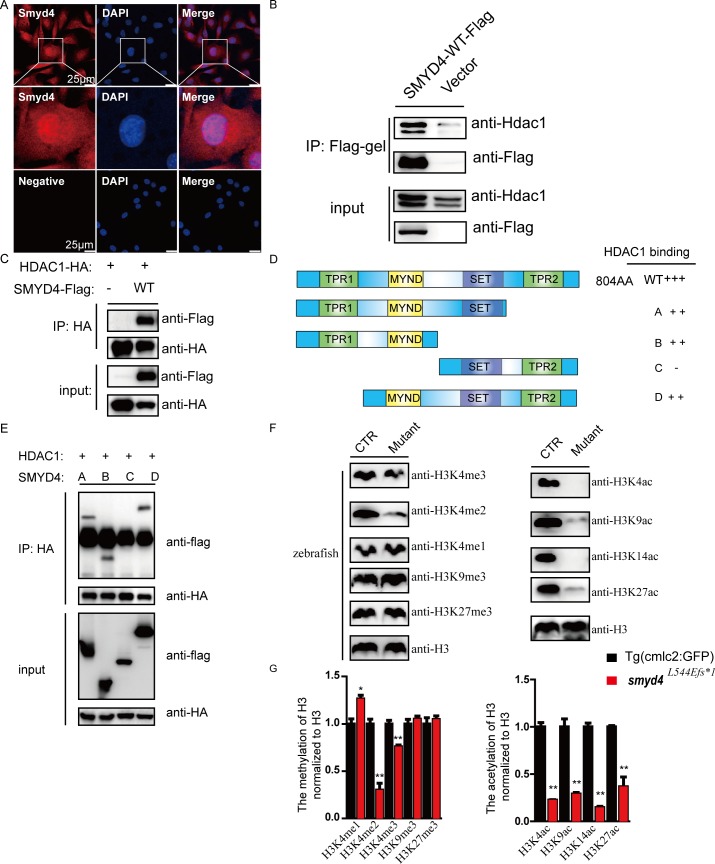
As shown in Fig 5A, SMYD4 was localized to both the nucleus and cytoplasm. To determine the biochemical function of SMYD4, flag-tagged SMYD4 (SMYD4flag) was first overexpressed in the HL-1 mouse cardiomyocyte cell line, followed by immunoprecipitation and mass spectrometric analysis to identify its interacting proteins. HDAC1 was identified as one of the major proteins to interact with SMYD4 (Fig 5B). This finding was further confirmed by Co-IP/western blotting analysis using a HEK293T cell line in which both SMYD4flag and HA-tagged HDAC1 (HDAC1HA) were co-overexpressed (Fig 5C). There are four functional domains in SMYD4. Two TPR domains are located at the N- and C- termini, an MYND domain can mediate interactions with partner proteins, and a SET domain functions as a methyltransferase. To determine the functional domain that was responsible for the SMYD4/HDAC1 interaction, as shown in Fig 5D and 5E, we generated several mutations in SMYD4 with different combinations of deletions in the SMYD4 protein and co-expressed these mutants with HDAC1 in HEK293T cells. We were able to use Co-IP/western blotting assays to demonstrate that the MYND domain was responsible for the interaction between SMYD4 and HDAC1.
Fig 5. SMYD4 interacts with HDAC1.
(A) Immunofluorescence staining and representative confocal images of the sub-cellular localization of SMYD4 in HL-1 cells. SMYD4 was found in both the nucleus and cytoplasm. The experimental groups without primary anti-SMYD4 antibody incubation served as negative controls; (B) Co-IP/western blotting analysis to confirm the interaction between SMYD4 and HDAC1. SMYD4flag was overexpressed in HL-1 cells. The cell extracts were immunoprecipitated with an anti-FLAG affinity gel, followed by western blotting analysis; (C) The interaction between SMYD4 and HDAC1 was further confirmed by Co-IP/western blotting analysis in HEK293T cells; (D-E) Using a Co-IP/western blotting assay to map the domains in SMYD4 that are responsible for the interaction with HDAC1. The MYND domain of SMYD4 was found to be critical for this interaction; (F) Aberrant histone modifications in MZsmyd4L544Efs*1 embryos at 48 hpf. Specifically, H3K4me2 and H3K4me3 were significantly reduced, and H3K4me was increased, while H3K9me3 and H3K27me3 were not affected, suggesting that SMYD4 is a H3K4-specific methyltransferase. H3K4ac, H3K9ac, H3K14ac, and H3K27ac were significantly abolished in MZsmyd4L544Efs*1 mutants, suggesting the increased activity of HDAC1 in MZsmyd4L544Efs*1 mutants and confirming SMYD4 as an important negative regulator of HDAC1 function; (G) The semi-quantitative analysis of the histone modifications changes in MZsmyd4L544Efs*1 mutants. (p<0.05 *, p<0.01 **).
To confirm the biochemical activities of SMYD4 as a methyltransferase and as a functional partner of HDAC1 in histone modification, we analyzed the changes in both histone methylation and acetylation modifications in the MZsmyd4L544Efs*1 embryos. As shown in Fig 5F and 5G, di- (me2) and tri-methylation (me3) at the lysine 4 site (K4) of histone 3 (H3) were significantly reduced, and mono-methylation of lysine 4 (H3K4me) was increased, while other lysine residues (i.e., K9 and K27) were not affected, suggesting that SMYD4 was specifically involved in H3K4 methylation. Interestingly, the acetylation of lysines 4, 9, 14, and 27 was dramatically reduced in the MZsmyd4L544Efs*1 mutants examined at 48 hpf (Fig 5F and 5G). This finding suggested that SMYD4 is a critical part of the HDAC1 functional complex and may function as an important negative regulator of HDAC1-mediated histone 3 modification and epigenetic regulation. Taken together, these data indicate that SMYD4 is a functional methyltransferase specific for H3K4 methylation and a functional partner of HDAC1 for regulating H3 acetylation.
Rare SMYD4 variants associated with CHD patients
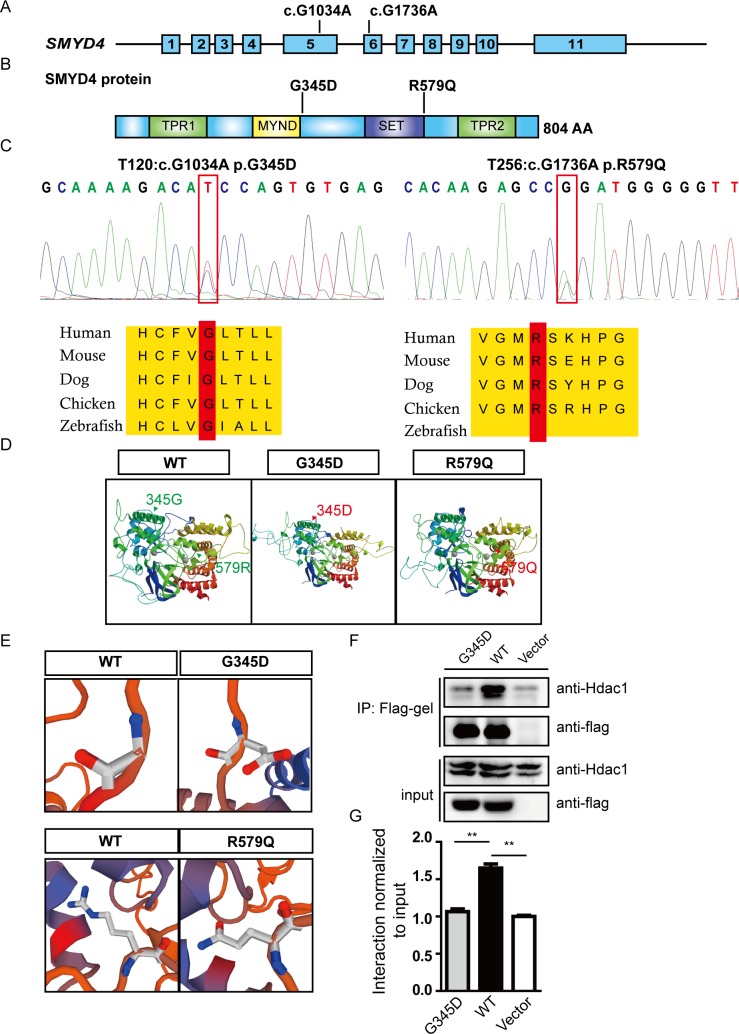
We used Target Exome Sequencing (TES) and screened a cohort of 208 patients with CHDs for potential genetic variants of SMYD4. The patient information is summarized in S1 Table and S2 Table. Two rare missense variants (c.1034G>A, p. G345D and c.1736G>A, p. R579Q) were identified and confirmed by Sanger sequencing in two individual patients (Fig 6A and 6B). The variants were not recorded in the 1000G database or in our internal whole-exome sequencing database generated from more than 3,500 patients without CHDs. The frequencies of G345D and R579Q in the EXAC database are 1/121,404 and 3/120,896, respectively. G345D was identified in a patient who was diagnosed with DCRV/VSD, and R579Q was identified in a patient with TOF (S3 Table). Both sites were evolutionally conserved from zebrafish to humans (Fig 6C). Both variants (G345D and R579Q) were predicted to be highly pathogenic and harmful by SIFT, PolyPhen2 and MutationTaster. Based on 3D-computational structure prediction using the SWISS MODEL, as shown in Fig 6D and 6E, both mutations led to significant changes in protein structure compared with that of the wild-type SMYD4. To confirm the pathogenicity of the variant SMYD4(G345D), Co-IP/western blotting assays were performed, and we found that the biochemical interaction between SMYD4(G345D) and HDAC1 was greatly attenuated compared to the interaction between SMYD4 and HDAC1 in HL-1 cells (Fig 6F and 6G), consistent with the predicted alteration of protein structure and function in SMYD4(G345D).
Fig 6. Rare variants in SMYD4 are associated with CHD patients.
(A) Schematic diagram of the human SMYD4 gene. SMYD4 is composed of eleven exons, and two rare missense variants (c. 1034G>A p.G345D and c. 1736G>A p.R579Q) located in exon 5 and 6, respectively, were identified in two CHD patients from a 208-patient cohort; (B) The variants G345D and R579Q are located at the edge of two functional domains, the MYND and SET domains, respectively; (C) Sanger sequencing confirmed the variants. Both variants were found at a site that is highly conserved from zebrafish to humans; (D) Predicted 3D structure of the wild-type and mutant SMYD4 proteins. The structures of both mutant proteins are significantly changed (The arrows pointing the location of the mutant amino acid); (E) Comparison of the structures at the mutation sites between wild-type SMYD4 and the two mutants. The structures of the mutant amino acids are completely changed; (F) Co-IP/western blotting assays revealed the attenuated interaction between SMYD4(G345D) and HDAC1 in HL-1 cells; (G) The semi-quantitative analysis of the Western blotting results demonstrated that the G345D mutant SMYD4 protein significantly disrupted the interaction with HDAC1 compared to the wild-type SMYD4 protein (p<0.01, **).
Functional confirmation of SMYD4(G345D) as a pathogenic mutation
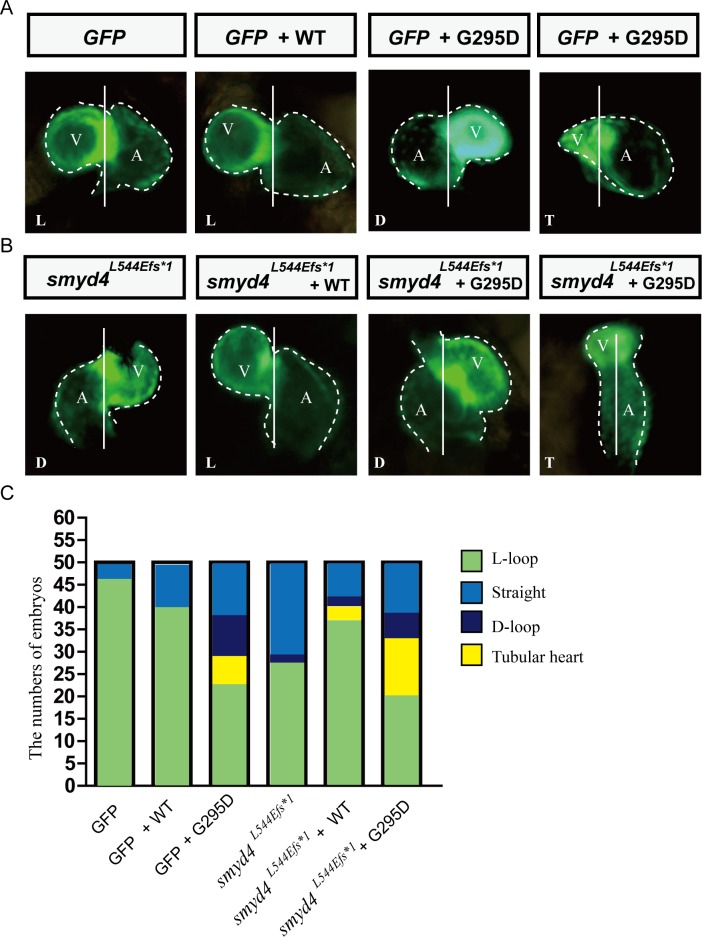
To confirm SMYD4(G345D) as a pathogenic mutation, we performed gain-of-function transgenic overexpression experiments by injecting wild-type smyd4 and smyd4(G295D) mRNA into normal Tg(cmcl2:GFP) embryos (Fig 7A and 7C). Based on the amino acid sequences of zebrafish smyd4 and human SMYD4, we generated smyd4(G295D) mutant cDNA, which was equivalent to human SMYD4(G345D) (Fig 6C and S7 Fig). We found that the smyd4(G295D) mRNA caused significantly more embryos with severe heart defects (e.g., D-loop and tubular hearts) than wild-type smyd4 mRNA (Fig 7A and 7C), suggesting that SMYD4(G345D) was harmful to cardiac development.
Fig 7. SMYD4(G345D) contributes to cardiac abnormalities.
(A) Representative cardiac morphology of wild-type embryos injected with either wild-type smyd4 or smyd4(G295D) mRNAs; (B) Representative cardiac morphology of MZsmyd4L544Efs*1 embryos injected with either wild-type smyd4 or smyd4(G295D); (C) Summary of the observations from (A) and (B). A total of 50 fish were screened.
To further confirm the effects of this mutation, we performed rescue experiments by analyzing and comparing the ability of mutant SMYD4(G345D) and wild-type SMYD4 to rescue the abnormal cardiac phenotypes of MZsmyd4L544Efs*1 mutants. We injected smyd4 wild-type and smyd4(G295D) mutant mRNAs into MZsmyd4L544Efs*1 single-cell embryos harvested from the homozygous breeding scheme described above. As shown in Fig 7B and 7C, wild-type smyd4 mRNA significantly reduced the number of embryos with malformed hearts compared to the number in the MZsmyd4L544Efs*1 mutant group, which indicates a partial but significant rescue phenotype for the wild-type smyd4 mRNA. In contrast, the smyd4(G295D) mRNA not only failed to rescue MZsmyd4L544Efs*1 but also significantly increased the number of embryos with malformed hearts and severe cardiac defects, such as tubular hearts (Fig 7B and 7C), further confirming that the smyd4(G295D) mutation is pathogenic. Taken together, our data implied that human SMYD4(G345D) was deleterious for heart development and a CHD-causing genetic variant.
Abnormal cardiac gene expression profiling of MZsmyd4L544Efs*1 hearts
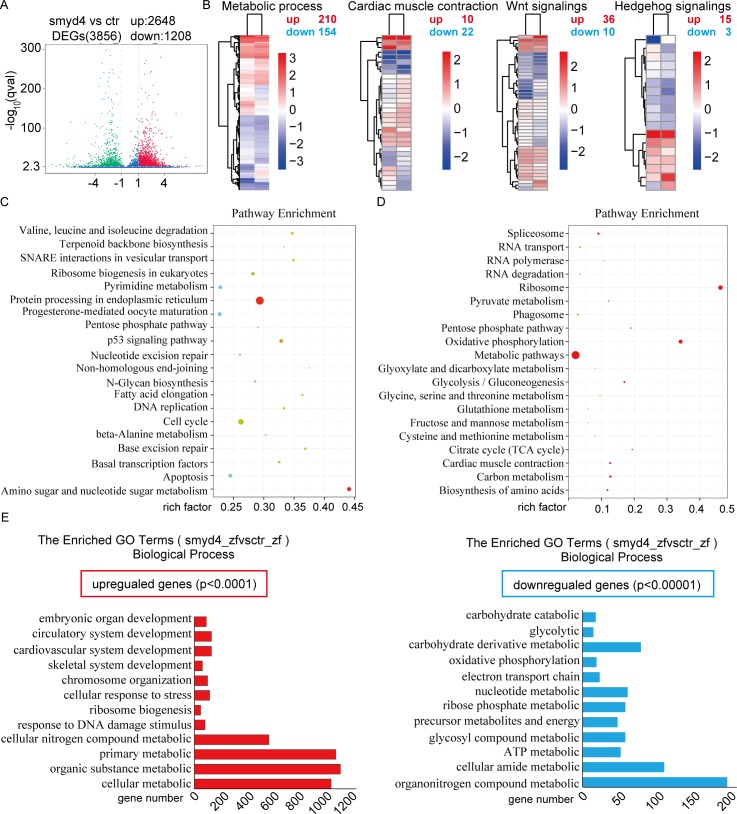
Given the severe cardiac defects and abnormal histone modifications in the MZsmyd4L544Efs*1 embryos, we anticipated a large number of gene alterations in MZsmyd4L544Efs*1 mutant hearts. We performed RNA-seq analysis, comparing normal and MZsmyd4L544Efs*1 hearts harvested at 72 hpf. As shown in Fig 8, a total of 3,856 differentially expressed (DE) genes were identified in MZsmyd4L544Efs*1 hearts. Among those genes, 2,648 genes were upregulated, and 1,208 genes were downregulated (Fig 8A). Not surprisingly, some important genes that were highly relevant to cardiac development were altered, which included the genes involved in cardiac muscle contraction (upregulated: 10; downregulated: 22) and key cardiac signaling pathways, such as the canonical Wnt signaling pathway (upregulated: 36, downregulated: 10) and the Hedgehog signaling pathway (upregulated: 15, downregulated: 3) (Fig 8B). To investigate whether this altered transcriptional profile was associated with specific pathways or biological processes, we performed KEGG pathway analysis, which indicated that the upregulated genes were enriched in the endoplasmic reticulum-mediated protein processing pathway in the Biological Process domain (Fig 8C) and the downregulated genes were enriched in several metabolic pathways, including carbon metabolism and the glycolysis/gluconeogenesis pathway (Fig 8D). Gene ontology (GO) analysis of the upregulated genes also revealed major terms in cellular metabolic processes, in which 975 genes were involved. A GO term analysis of the downregulated genes revealed the enrichment of a large number of genes in the organonitrogen compound metabolic process (185 genes), the ATP metabolic process (49 genes) and the glycosyl compound metabolic process (55 genes) (Fig 8E). This finding provided an important hint that smyd4/hdac1-mediated epigenetic regulation likely occurred via the control of endoplasmic reticulum-mediated protein processing and several key metabolic pathways in the heart during zebrafish development.
Fig 8. RNA-seq and bioinformatics analysis of MZsmyd4L544Efs*1 hearts.
RNA-seq analyses were performed with Tg(cmcl2:GFP) and MZsmyd4L544Efs*1 hearts at 72 hpf; (A) A volcano plot demonstrated that a total of 3,856 genes were differentially expressed in MZsmyd4L544Efs*1 hearts (upregulated: 2,648, downregulated: 1,208); (B) A heat-map showed that genes involved in cardiac development signaling pathways, including Wnt signaling (upregulated: 36, downregulated: 10), Hedgehog signaling (upregulated: 15, downregulated: 3), the metabolism pathway (upregulated: 210, downregulated: 154), and cardiac muscle contraction (upregulated: 10, downregulated: 22), were dysregulated in MZsmyd4L544Efs*1 hearts; (C) KEGG pathway analysis revealed a major enrichment of upregulated genes in the endoplasmic reticulum-mediated protein processing pathway; (D) KEGG pathway analysis revealed an enrichment of downregulated genes in pathways related to metabolic processes and oxidative phosphorylation; (E) Gene Ontology (GO) terms of the biological processes (BP) associated with all differentially expressed genes. The up-regulated genes function in organic substance metabolic processes, cardiovascular system development and embryonic organ development (p < 0.0001), while the downregulated genes were enriched in the carbohydrate metabolic process, the glycolytic process, and the ATP metabolic process (p < 0.00001).
Discussion
SMYDs are a family of unique lysine-histone methyltransferases that contain the well-conserved SET and MYND domains, as well as two TPR domains. The biological functions of SMYDs are largely unknown. By generating smyd4 mutant zebrafish using the CRISPR/Cas9 technology, we have shown that smyd4 is indispensable for cardiac development. This finding is consistent with the observation that ubiquitously expressed smyd4 in early zebrafish embryos becomes more enriched to zebrafish developing hearts at 48 hpf during embryogenesis, despite the fact that gross expression levels are reduced dramatically at this stage, which is followed by a quick up-regulation of smyd4 expression in embryos at 72 hpf (Fig 1A). These are critical stages, in which the newly formed heart switches from the cardiac morphogenic pathway to cardiac maturation pathways to eventually form a normal functional heart.
There are several major cardiac defects in the MZsmyd4L544Efs*1 mutants, including the defects in left-right patterning and looping, and the hypoplastic ventricular walls in the developing hearts. Furthermore, the cell proliferative activities in MZsmyd4L544Efs*1 developing ventricles are significantly decreased, which likely leads to the hypoplastic ventricle. In adults, the thickening of the ventricular compact wall that is seen in some mutant hearts is likely a maladaptation of compromised cardiac function due to cardiac developmental defects.
Considering smyd4 shares similar protein structure and enzymatic activities with other SMYD family members, genetic and functional redundancy may occur in MZsmyd4L544Efs*1 mutants. It has been shown that smyd1b mutants display a similar pericardial edema and congestive blood circulation [24]. The morpholino knock-down of another SMYD family member smyd3 in zebrafish embryos also produces cardiac looping defects [13]. Both of these prior studies suggest potential genetic interactions and/or redundancies among these family members in zebrafish heart development. Some variations in the cardiac defects as well as certain degrees of varying severity of cardiac defects among the mutant embryos further support this notion. However, it is also clear that each member of SMYD family has its unique function. For example, myofibril disorganization in skeletal muscle are only seen in smyd1b mutants [24]. We have not observed any such a defect in MZsmyd4L544Efs*1 mutants, suggesting that the function of smyd4 is more relevant to cardiac development in zebrafish.
Our observations strongly suggest that smyd4 plays an important function in cardiac development. This conclusion is also supported by our human genetic study, which identified two rare SMYD4 variants in a 208-patient cohort with CHDs. The data obtained after the overexpression of mutant smyd4(G295D) and from rescue experiments in which smyd4(G295D) mRNA was injected into MZsmyd4L544Efs*1 mutants strongly indicates that the human variant SMYD4(G345D) is pathogenic, further suggesting SMYD4 as a genetic contributor to CHDs. This finding indicates the importance of including SMYD4 in CHD genetic screening panels in the future.
One of our key findings is that SMYD4 interacts with the major histone modification enzyme and epigenetic regulator HDAC1 via the well-conserved MYND domain. Our finding is consistent with the previous finding that dSmyd4 can interact with dHDAC1 in Drosophila muscle development [15]. The MYND domain is known for its role in protein-protein interactions and was previously shown to recruit the HDAC complex to regulate gene expression [25]. The mutation in SMYD4(G345D) is located at the edge of the MYND domain and between the MYND and SET domains. Our biochemical data show that SMYD4(G345D) has a dramatically reduced ability to interact with HDAC1. Although the TPR domains are still poorly understood, previous studies suggest that the C-terminal TPR2 domain is vital for methyltransferase activity and protein-protein interactions [3,26]. The TPR2 domain of SMYD2 is indispensable for its interaction with HSP90, which proved to be critical for titin filament organization [12,27]. Currently, we are in the process of generating smyd4-tpr2del mutant zebrafish similarly using the CRISPR/Cas9 technology to further investigate the role of TPR2 in smyd4 biological function.
SMYDs 1 and 3 catalyze mono-, di-, and trimethylation of H3K4 [11,28]. Similarly, SMYD2 can mono-methylate H3K4 (H3K4me) and di-methylate H3K26 (H3K26me2) and p53K37me [6,29]. In MZsmyd4L544Efs*1 mutants, H3K4me2 and H3K4me3 are reduced, suggesting that smyd4 is a specific methyltransferase for H3K4 methylation. Notably, H3K4ac, K9ac, K14ac, and K27ac were all abolished in the MZsmyd4L544Efs*1 mutants. This finding implies that the deficiency of smyd4 impacts the function of hdac1 (the gene homologous to both HDAC1 and HDAC2 in mammals). As previously demonstrated, cardiac-specific deletion of the mouse Hdac1 and Hdac2 genes evoked a strong heart failure phenotype [20], which is consistent with our finding. We are currently using biochemical approaches to determine whether smyd4 serves as a simple docking protein to provide chaperone functions for hdac1 or functions as a key modulating molecule for hdac1. Nevertheless, our data suggest that smyd4 plays a critical role in the epigenetic regulation of gene expression via its dual activities as a methyltransferase and negative regulator of hdac1.
RNA-seq analysis comparing wild-type and MZsmyd4L544Efs*1 mutant hearts demonstrates that the expression of over 3,000 genes is altered, which may reflect the potential function of smyd4’s broad epigenetic regulation of its target genes. In addition to genes related to cardiac muscle contraction and cardiac signaling pathways that are highly relevant to cardiogenesis (e.g., the canonical Wnt and Hedgehog signaling pathways) (Fig 8), our KEGG pathway and GO annotation analyses of altered genes revealed an overwhelming enrichment in several cellular metabolic pathways, including the endoplasmic reticulum-mediated protein processing pathway. This finding is very different from the publicly available RNA-seq database for zebrafish heart developmental defects [30–32]. This finding suggests that smyd4 has a unique and specific biological function in regulating cellular metabolism. However, as it is technically difficult to perform ChIP-seq on zebrafish embryonic hearts, we cannot currently determine which specific components of the pathways are primarily affected and which are affected secondarily. Our future study will switch to a mammalian system to determine the detailed molecular mechanism by which SMYD4 modulates cellular metabolism or signaling pathways via its important role in epigenetic regulation. We will re-evaluate whether these altered metabolic pathways play critical roles in cardiac development and the cardiac defects seen in smyd4 mutant embryos.
This study is the first characterization of SMYD4 in vertebrate development and physiological function. Taken together, our results demonstrate the critical role of smyd4 in embryonic development and heart formation. Our data suggest that smyd4 functions as a histone methyltransferase and, by interacting with HDAC1, also serves as a potential modulator for histone acetylation. In addition, our work has also provided genetic and functional evidences that rare SMYD4 variants likely contribute to CHDs.
Materials and methods
Ethics statement
All genetic studies were approved by the Ethics Committee of the Children’s Hospital of Fudan University, China. The approval number is: [2015]92). All patients provided written informed consent in accordance to the Declaration of Helsinki. The Research Ethics Committee of the Children’s Hospital of Fudan University, China, approved and monitored all zebrafish procedures following the guidelines and recommendations outlined by the Guide for the Care and Use of Laboratory Animals. The approval number is: [2015]92). For all experiments, wild-type zebrafish embryos of the Tu and transgenic Tg (cmcl2:GFP) (cardiac myosin light chain 2:GFP reporter) strains were used.
In situ hybridization
The expression of smyd4 was detected in zebrafish embryos from the 10 to 72 hpf stages using a smyd4-specific antisense probe. The template for the smyd4 probe was amplified from the cDNA of zebrafish at 24 hpf. The 508-bp fragment, which was obtained using specific primers (smyd4-probe-F: GAAGTGTGTGAAATGTGGAAAGCCTCTT and smyd4-probe-R: TTCACTCAGTTCCTGCAGTTCTTCACAG), was cloned into the pEasy-T vector (Promega, USA). After linearization of the plasmids, the antisense and sense probes were transcribed and labelled with digoxigenin in vitro. RNA in situ hybridization was performed as described previously [33]. Briefly, zebrafish embryos at different stages were collected and fixed in 4% paraformaldehyde at 4°C overnight. Embryos older than 24 hpf were digested by proteinase K at room temperature. Then, the embryos were pro-hybridized at 65°C for 4 hours and subsequently incubated with the antisense or sense probes overnight. An anti-digoxigenin antibody (Roche, USA) was used to bind the probes overnight at 4°C. Finally, the embryos were stained with NBT/BCIP (Vector, USA) and photographed in methylcellulose using a Leica M205C microscope.
Zebrafish RNA preparation and qRT-PCR
Embryos of the wild-type Tu zebrafish strain were collected at 0.2, 2, 4, 8, 10, 24, 48 and 72 hpf. The total RNA was extracted using the TRIzol reagent (Invitrogen, USA) and converted to cDNA using the PrimeScript RT Reagent Kit (Takara Bio, Japan). The real-time qPCR reactions were performed with SYBR Premix Ex Taq (Takara Bio, Japan) using the Roche 480 plus system (Roche, USA). The real-time primers for the zebrafish are summarized in S4 Table.
Generation of smyd4 mutant zebrafish lines
smyd4 target sites were designed using the website http://zifit.partners.org/ZiFiT/CSquare9Nuclease.aspx. The provided sites were then screened in Ensemble. Three sites that specifically recognize the sequence of smyd4 in the zebrafish genome were chosen for the interruption of smyd4. sgRNA1 (GGAGTAATGAAGCACTGCTG), sgRNA2 (GGAGCTGATCTGCTGGCCAT), and sgRNA3 (GGAGCGTCAGCGCCTCCTGC) targeted exons 2, 4 and 6 of smyd4, respectively. We cloned these sites into the gRNA plasmid p-T7-gRNA, which was provided by Professor Li Qiang. gRNAs were transcribed in vitro using the MAXIscript T7 kit (Ambion, USA). Cas9 mRNA was transcribed from the pSP6-2Snls-spCas9 plasmid using the SP6 mMESSAGE mMACHINE Kit (Ambion, USA), and poly A tails were added using the poly A Tailing Kit (Ambion, USA). All gRNAs and the Cas9 mRNA were purified and dissolved in nuclease-free water before injection using the mirVana miRNA Isolation Kit (Ambion, USA) and the RNA Purification Kit (TIANGEN, China). gRNA and Cas9 mRNA were co-injected into the embryos at the single-cell stage. Twenty injected embryos were used to identify the efficiency, and the remaining embryos were raised to adulthood to obtain the mosaic founders. These mosaic fish were crossed with wild-type zebrafish to produce heterozygotes, which were genotyped using Sanger sequencing methods. To analyze the cardiac defects in MZsmyd4L544Efs*1 mutants, we crossed the smyd4L544Efs*1 line to the cardiomyocyte-specific transgenic reporter line Tg (cmcl2:GFP). All primers and target sites of smyd4 are summarized in S4 Table.
Cardiac phenotype analysis of the MZsmyd4 L544Efs*1 line
Embryos were collected at 48, 72, and 96 hpf for phenotype analysis. The embryos were fixed in methylcellulose and imaged using Leica M205C and Leica SP8 microscopes (Leica, Germany). To determine the number of cardiomyocytes in the developing ventricle, the embryonic hearts at 96 hpf were carefully collected and scanned using Leica SP8 confocal microscope. The z-step was set at 1μm. The images with largest section of ventricles were chosen for the analysis. DAPI and EGFP double positive cells were scored for cardiomyocyte. Adult fish at 6 months of age were photographed to record the body size and the developmental states of different organs, including the head, eyes, fins, and tails. These fish were dissected after anesthesia. The hearts of adult zebrafish were fixed and photographed in 4% paraformaldehyde. Serial sectioning with H&E staining was performed for these heart samples.
Proliferation and apoptosis assays
Embryos were collected at 48 hpf, fixed in 4% paraformaldehyde at 4°C overnight. For the proliferation assay, the embryos were digested using Collagenase, Type II (Life technologies, USA) at room temperature, and blocking was performed for one hour at room temperature, and followed by incubating with the primary antibody against p-H3(S10) (Abacam, USA) or PCNA (Genetex, USA) at 4°C overnight. Second antibody were from the series of Alexa Fluor (Life technologies, USA). For the apoptosis assay, In Situ Cell Death Detection kit, TMR red (Roche, USA) was used and all procedures were performed as the instruction manual described. Finally, embryo hearts were collected under a Leica M205C stereomicroscope and were imaged using a Leica SP8 microscope.
Plasmid construction and protein-protein interaction identification
A plasmid containing wild-type human SMYD4(BC035077) was obtained from Abmgood (Abmgood, USA). Then, Flag-tagged wild-type SMYD4 and HA-tagged wild-type HDAC1 were cloned into the expression plasmid. The mutation (G345D) identified in patients was obtained using the KOD-Plus Mutagenesis Kit (Toyobo, Japan). All plasmids were confirmed via Sanger sequencing. The anti-SMYD4 (Proteintech, USA), anti-HDAC1 (Proteintech, USA), anti-Flag (Abmart, China), and anti-HA (Abmart, China) antibodies were used. Wild-type SMYD4 was overexpressed in HL-1 cells. After 48-h transfections, cell lysates were obtained in RIPA containing 1 mM PMSF and complete protease inhibitors (Roche, USA). Immunoprecipitation was performed using an anti-Flag affinity gel (Biotool, USA). SDS-PAGE was performed to resolve the eluates. After sliver staining, the proteins underwent mass spectrometry analysis. Protein-protein interactions were verified in HL-1 cells after transient overexpression of SMYD4. Co-immunoprecipitation was performed to confirm the interaction between SMYD4 and HDAC1 in HEK293T cells using anti-tag antibodies.
Domain mapping and mutant protein analysis
HEK293T cells were transiently co-transfected with pCDH-Flag-SMYD4 deletion mutants and pcDNA3-HA-HDAC1. Plasmids of SMYD4 were co-transfected into HEK293T cells, and cell extracts were prepared as described above. Immunoprecipitations were performed with anti-HA affinity beads (Biotool, USA). The beads were washed five times, and bound proteins were eluted in SDS-PAGE loading buffer and analyzed via western blotting.
Immunofluorescence
Briefly, the immunofluorescence process is described as follows. The cells were fixed in 4% paraformaldehyde and then underwent cell permeation and blocking. The antibody used for immunofluorescence was anti-SMYD4 (Proteintech, USA). Primary antibodies were incubated overnight at 4°C. Secondary antibodies were from the Alexa Fluorescence Series (Life technologies, USA). Finally, the cells were imaged in Diamond anti-fade agent (Life technologies, USA) using a Leica SP8 microscope.
Zebrafish western blotting and antibodies
Tg(cmcl2:GFP) and MZsmyd4L544Efs*1 embryos were collected at 48 hpf. Protein was obtained in RIPA containing 1 mM PMSF and complete protease inhibitors (Roche, USA) after sonication. The histone modification antibodies used in this study include anti-H3 (CST, USA), anti-H3K4ac (Active Motif, USA), anti-H3K9ac (Active Motif, USA), anti-H3K14ac (Active Motif, USA), anti-H3K27ac (Active Motif, USA), anti-H3K4me1 (Active Motif, USA), anti-H3K4me2 (Active Motif, USA), anti-H3K4me3 (Abcam, USA), anti-H3K9me3 (Abcam, USA), and anti-H3K27me3 (Millipore, USA).
Human samples and ethics statement
This study was approved by the research ethics committee of the Children’s Hospital of Fudan University in Shanghai, China (number: [2015]92). The diagnosis of CHD patients was based on echocardiography at the Children’s Hospital of Fudan University in Shanghai, China. All patients involved in this research had not been diagnosed with extra cardiac anomalies and did not have common chromosomal anomalies, such as the 22q11 microdeletion. Human cardiac tissue samples from CHD patients were obtained from the Biobank of the Children’s Hospital of Fudan University in Shanghai, China. Cardiac tissues were removed from the blocked right ventricular outflow tract during surgery. All tissue samples were maintained in RNAlater RNA Stabilization Solution (Thermo Scientific, USA) after surgery and stored at -80°C before use. RNA was extracted from the tissue samples using the Trizol reagent (Invitrogen, USA) and immediately underwent reverse transcription using the PrimeScript RT Reagent Kit (Takara, Japan). All detailed patient information is summarized in S2 Table.
Human genomic sequencing studies and in silico analysis
The peripheral venous blood samples from 113 patients were prepared for DNA extraction using the Blood Extraction Kit (QIAGEN, Germany). The cardiac tissue samples from 95 patients were prepared for cDNA extraction. All of the regions covered by TES and all primers for smyd4 exon sequencing in cDNA are listed in S5 Table. Variant analysis was performed using the Mutation Surveyor software (Softgenetics, USA). All variants were screened in public databases, including the 1000 Genome database, the dbSNP database, and the ExAC database, and an internal database in the molecular diagnosis laboratory at the Children’s Hospital of Fudan University. A risk analysis of SNVs was performed using SIFT, Polyphen2, and Mutation Taster to predict the possible effects on protein function. A 3D structure analysis of the wild-type and mutant proteins was performed on the SWISS MODEL website (https://www.swissmodel.expasy.org/).
Microinjection of wild-type or mutant smyd4 mRNA (G295D) and phenotype analysis
Based on its pathogenic prediction, the G345D SMYD4 variant was selected for mutation analysis. Homology analysis showed that the mutant G295D in smyd4 was equivalent to the mutant G345D in SMYD4. The template for smyd4 wild-type mRNA transcription was amplified from zebrafish cDNA at 24 hpf. The template of the mutant (G295D) in smyd4 transcription was obtained using the KOD-Plus Mutagenesis Kit (Toyobo, Japan). The wild-type and mutant mRNAs of smyd4 were transcribed using the mMessage mMachine T7 Ultra Kit (Ambion, USA), and poly A tails were added using the poly A Tailing Kit (Ambion, USA), according to the instruction manual. The mRNAs were resolved in nuclease-free water and finally quantified to 150 ng/μl for microinjection. Embryos of Tg(cmcl2:GFP) and MZsmyd4 L544Efs*1 were collected. A total of 3 nl of mRNA was microinjected into embryos at the single-cell stage. Fifty embryos from each group with cardiac GFP at 48 hpf were chosen for phenotype analysis. The embryos were fixed in 3% methylcellulose after anesthetization and then observed for cardiac morphology using a Leica M205C stereomicroscope.
RNA sequencing
Heart tissue was collected from 50 MZsmyd4L544Efs*1 or Tg(cmcl2:GFP) embryos. Cardiac-specific GFP helped us successfully obtain embryo heart tissue. Embryo hearts were collected at 72 hpf after anesthesia using a Leica M205C stereomicroscope. In-depth RNA sequencing was performed by the Novogene Experimental Department in China. The raw sequencing image data were examined via the Illumina analysis pipeline and aligned with the unmasked zebrafish reference genome. Differential expression analysis of the two groups was performed using the DESeq2 R package (1.10.1). The resulting P-values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with an adjusted P-value < 0.005 and |log2 (Fold change)|>1 found by DESeq2 were assigned as differentially expressed.
Statistical analyses
The Student’s t-test was used for all statistical analyses. A p-value of < 0.05 (2-sided) was regarded as statistically significant. All experiments were repeated three times. All data were analyzed with GraphPad Prism (version 5.0).
Supporting information
An 11-nt insertion was achieved using the CRISPR-Cas9 technology and was validated by cDNA PCR and sequencing analyses in MZsmyd4L544Efs*1.
(TIF)
(TIF)
The confocal images of the largest section of the ventricle of MZsmyd4 L544Efs*1 and Tg(cmcl2:GFP) hearts at 96 hpf.
(TIF)
(TIF)
TUNEL assays showed that there is no apoptosis in neither control hearts and MZsmyd4L544Efs*1 hearts (Upper panel). The positive TUNEL signals (red fluorescence) in the tail fin and embryos at 48 hpf treated with DNase I were used as positive controls for the analysis (Lower panel).
(TIF)
The skeletal muscle structures of MZsmyd4L544Efs*1 mutants appeared to be normal at 72 hpf.
(TIF)
Both human SMYD4 and the zebrafish smyd4 protein have four functional domains, including 2 TPR domains, one MYND domain, and one SET domain. These domains are highly conserved between the two species. The two rare variants identified in CHD patients are located at the edge of the MYND and SET domains. Zebrafish smyd4(G295D) is equivalent to human SMYD4(G345D).
(TIF)
(DOCX)
(XLSX)
(DOCX)
(XLSX)
(XLSX)
(MP4)
(MP4)
Acknowledgments
We would like to thank the Bio-bank and Pediatric Heart Center of the Children’s Hospital of Fudan University for providing the research samples, and we also thank all patients who were involved in this study. We thank the staff of the Shanghai Key Lab of Birth Defects and the Zebrafish Facility at Children’s Hospital of Fudan University. We also thank Prof. Fei Lan for advice on this study.
Data Availability
Raw and normalized RNA-seq data have been deposited in the NCBI-GEO database under the accession code GSE109553. All other relevant data are available within the manuscript and its Supporting Information files.
Funding Statement
This work was supported in part by the National Key Research and Development Program (2016YFC1000500), the National Natural Science Foundation of China (81570283, 81570286, 81470446, and 31401237) and the National Institute of Health P01HL134599-01. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jenuwein T, Laible G, Dorn R, Reuter G (1998) SET domain proteins modulate chromatin domains in eu- and heterochromatin. Cell Mol Life Sci 54: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews JM, Bhati M, Lehtomaki E, Mansfield RE, Cubeddu L, et al. (2009) It takes two to tango: the structure and function of LIM, RING, PHD and MYND domains. Curr Pharm Des 15: 3681–3696. [DOI] [PubMed] [Google Scholar]
- 3.Sirinupong N, Brunzelle J, Ye J, Pirzada A, Nico L, et al. (2010) Crystal structure of cardiac-specific histone methyltransferase SmyD1 reveals unusual active site architecture. J Biol Chem 285: 40635–40644. 10.1074/jbc.M110.168187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, Cheung T, Grande C, Ferguson AD, Zhu X, et al. (2011) Biochemical characterization of human SET and MYND domain-containing protein 2 methyltransferase. Biochemistry 50: 6488–6497. 10.1021/bi200725p [DOI] [PubMed] [Google Scholar]
- 5.Cho HS, Hayami S, Toyokawa G, Maejima K, Yamane Y, et al. (2012) RB1 methylation by SMYD2 enhances cell cycle progression through an increase of RB1 phosphorylation. Neoplasia 14: 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MA, Sims RJ 3rd, Gottlieb PD, Tucker PW (2006) Identification and characterization of Smyd2: a split SET/MYND domain-containing histone H3 lysine 36-specific methyltransferase that interacts with the Sin3 histone deacetylase complex. Mol Cancer 5: 26 10.1186/1476-4598-5-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diehl F, Brown MA, van Amerongen MJ, Novoyatleva T, Wietelmann A, et al. (2010) Cardiac deletion of Smyd2 is dispensable for mouse heart development. PLoS One 5: e9748 10.1371/journal.pone.0009748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan D, Rasmussen TL, Nakagawa O, McAnally J, Gottlieb PD, et al. (2005) BOP, a regulator of right ventricular heart development, is a direct transcriptional target of MEF2C in the developing heart. Development 132: 2669–2678. 10.1242/dev.01849 [DOI] [PubMed] [Google Scholar]
- 9.Proserpio V, Fittipaldi R, Ryall JG, Sartorelli V, Caretti G (2013) The methyltransferase SMYD3 mediates the recruitment of transcriptional cofactors at the myostatin and c-Met genes and regulates skeletal muscle atrophy. Genes Dev 27: 1299–1312. 10.1101/gad.217240.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlieb PD, Pierce SA, Sims RJ, Yamagishi H, Weihe EK, et al. (2002) Bop encodes a muscle-restricted protein containing MYND and SET domains and is essential for cardiac differentiation and morphogenesis. Nat Genet 31: 25–32. 10.1038/ng866 [DOI] [PubMed] [Google Scholar]
- 11.Tan X, Rotllant J, Li H, De Deyne P, Du SJ (2006) SmyD1, a histone methyltransferase, is required for myofibril organization and muscle contraction in zebrafish embryos. Proc Natl Acad Sci U S A 103: 2713–2718. 10.1073/pnas.0509503103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voelkel T, Andresen C, Unger A, Just S, Rottbauer W, et al. (2013) Lysine methyltransferase Smyd2 regulates Hsp90-mediated protection of the sarcomeric titin springs and cardiac function. Biochim Biophys Acta 1833: 812–822. 10.1016/j.bbamcr.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 13.Fujii T, Tsunesumi S, Yamaguchi K, Watanabe S, Furukawa Y (2011) Smyd3 is required for the development of cardiac and skeletal muscle in zebrafish. PLoS One 6: e23491 10.1371/journal.pone.0023491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii T, Tsunesumi S, Sagara H, Munakata M, Hisaki Y, et al. (2016) Smyd5 plays pivotal roles in both primitive and definitive hematopoiesis during zebrafish embryogenesis. Sci Rep 6: 29157 10.1038/srep29157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson EC, Travers AA (2008) A Drosophila Smyd4 homologue is a muscle-specific transcriptional modulator involved in development. PLoS One 3: e3008 10.1371/journal.pone.0003008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierpont ME, Basson CT, Benson DW Jr., Gelb BD, Giglia TM, et al. (2007) Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 115: 3015–3038. 10.1161/CIRCULATIONAHA.106.183056 [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Villalba A, Hoppler S, van den Hoff MJ (2016) Wnt signaling in the heart fields: Variations on a common theme. Dev Dyn 245: 294–306. 10.1002/dvdy.24372 [DOI] [PubMed] [Google Scholar]
- 18.Luxan G, D'Amato G, MacGrogan D, de la Pompa JL (2016) Endocardial Notch Signaling in Cardiac Development and Disease. Circ Res 118: e1–e18. 10.1161/CIRCRESAHA.115.305350 [DOI] [PubMed] [Google Scholar]
- 19.Zhang QJ, Liu ZP (2015) Histone methylations in heart development, congenital and adult heart diseases. Epigenomics 7: 321–330. 10.2217/epi.14.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, et al. (2007) Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev 21: 1790–1802. 10.1101/gad.1563807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, et al. (2013) De novo mutations in histone-modifying genes in congenital heart disease. Nature 498: 220–223. 10.1038/nature12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin SC, Homsy J, Zaidi S, Lu Q, Morton S, et al. (2017) Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet 49: 1593–1601. 10.1038/ng.3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Roeck A, Van den Bossche T, van der Zee J, Verheijen J, De Coster W, et al. (2017) Deleterious ABCA7 mutations and transcript rescue mechanisms in early onset Alzheimer's disease. Acta Neuropathol 134: 475–487. 10.1007/s00401-017-1714-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Just S, Meder B, Berger IM, Etard C, Trano N, et al. (2011) The myosin-interacting protein SMYD1 is essential for sarcomere organization. J Cell Sci 124: 3127–3136. 10.1242/jcs.084772 [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Chen W, Gaudet J, Cheney MD, Roudaia L, et al. (2007) Structural basis for recognition of SMRT/N-CoR by the MYND domain and its contribution to AML1/ETO's activity. Cancer Cell 11: 483–497. 10.1016/j.ccr.2007.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sirinupong N, Brunzelle J, Doko E, Yang Z (2011) Structural insights into the autoinhibition and posttranslational activation of histone methyltransferase SmyD3. J Mol Biol 406: 149–159. 10.1016/j.jmb.2010.12.014 [DOI] [PubMed] [Google Scholar]
- 27.Donlin LT, Andresen C, Just S, Rudensky E, Pappas CT, et al. (2012) Smyd2 controls cytoplasmic lysine methylation of Hsp90 and myofilament organization. Genes Dev 26: 114–119. 10.1101/gad.177758.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamamoto R, Furukawa Y, Morita M, Iimura Y, Silva FP, et al. (2004) SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nat Cell Biol 6: 731–740. 10.1038/ncb1151 [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Perez-Burgos L, Placek BJ, Sengupta R, Richter M, et al. (2006) Repression of p53 activity by Smyd2-mediated methylation. Nature 444: 629–632. 10.1038/nature05287 [DOI] [PubMed] [Google Scholar]
- 30.Mosimann C, Panakova D, Werdich AA, Musso G, Burger A, et al. (2015) Chamber identity programs drive early functional partitioning of the heart. Nat Commun 6: 8146 10.1038/ncomms9146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster K, Leeke B, Meier M, Wang Y, Newman T, et al. (2015) A neural crest origin for cohesinopathy heart defects. Hum Mol Genet 24: 7005–7016. 10.1093/hmg/ddv402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teittinen KJ, Gronroos T, Parikka M, Junttila S, Uusimaki A, et al. (2012) SAP30L (Sin3A-associated protein 30-like) is involved in regulation of cardiac development and hematopoiesis in zebrafish embryos. J Cell Biochem 113: 3843–3852. 10.1002/jcb.24298 [DOI] [PubMed] [Google Scholar]
- 33.Ma Z, Xia W, Liu F, Ma J, Sun S, et al. (2017) SLC44A4 mutation causes autosomal dominant hereditary postlingual non-syndromic mid-frequency hearing loss. Hum Mol Genet 26: 3234 10.1093/hmg/ddx232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An 11-nt insertion was achieved using the CRISPR-Cas9 technology and was validated by cDNA PCR and sequencing analyses in MZsmyd4L544Efs*1.
(TIF)
(TIF)
The confocal images of the largest section of the ventricle of MZsmyd4 L544Efs*1 and Tg(cmcl2:GFP) hearts at 96 hpf.
(TIF)
(TIF)
TUNEL assays showed that there is no apoptosis in neither control hearts and MZsmyd4L544Efs*1 hearts (Upper panel). The positive TUNEL signals (red fluorescence) in the tail fin and embryos at 48 hpf treated with DNase I were used as positive controls for the analysis (Lower panel).
(TIF)
The skeletal muscle structures of MZsmyd4L544Efs*1 mutants appeared to be normal at 72 hpf.
(TIF)
Both human SMYD4 and the zebrafish smyd4 protein have four functional domains, including 2 TPR domains, one MYND domain, and one SET domain. These domains are highly conserved between the two species. The two rare variants identified in CHD patients are located at the edge of the MYND and SET domains. Zebrafish smyd4(G295D) is equivalent to human SMYD4(G345D).
(TIF)
(DOCX)
(XLSX)
(DOCX)
(XLSX)
(XLSX)
(MP4)
(MP4)
Data Availability Statement
Raw and normalized RNA-seq data have been deposited in the NCBI-GEO database under the accession code GSE109553. All other relevant data are available within the manuscript and its Supporting Information files.