Abstract
Tardive dyskinesia (TD) is a devastating motor disorder associated with the etiological process of schizophrenia or antipsychotic medication treatments. To examine whether cerebral morphological changes may manifest in TD, we used voxel-based morphometry to analyze high-resolution T1-weighted brain structural magnetic resonance images from 32 schizophrenics with TD (TD group), 31 schizophrenics without TD (non-TD group), and 32 healthy controls (HC group). We also assessed psychopathological symptoms with the Positive and Negative Syndrome Scale (PANSS), and TD severity with the Abnormal Involuntary Movement Scale (AIMS). We compared gray matter volumes (GMVs) among groups, and tested for correlations between GMV changes and psychopathological symptoms or TD severity. The results showed significant differences in GMV in the frontal and temporal cortices, insula and cerebellum among the three groups. Brainstem and inferior frontal and precentral gyri GMVs were significantly larger, whereas cuneus and lingual gyrus GMVs were significantly smaller in the TD group as compared to non-TD group. Further, the cuneus and lingual gyrus GMVs were positively correlated with AIMS scores in the TD group. The current results suggest that TD may be associated with the alterations in GMV that are different from that of schizophrenics without TD. Further studies are needed to confirm and to examine the functional significance of these structural findings.
Introduction
Tardive dyskinesia (TD) is characterized by repetitive involuntary movements, usually involving the mouth, face, and tongue, and sometimes the limb and trunk musculature, as part of the etiological process of schizophrenia or side effects of antipsychotic medications. TD may occur from days to years after the initiation of antipsychotic treatment and persist even after a switch of medications to atypical antipsychotics1,2 or after complete withdrawal of medications3–6. TD reduces quality of life and disrupts a patient’s personal, social, and professional function7. A recent report showed that the annualized incidence of TD was 5.5% in patients treated with first-generation antipsychotics, and 3.5% in those treated with second-generation antipsychotics8,9. The incidence of TD with atypical antipsychotics is higher than expected, and underscores the fact that TD remains a significant clinical problem10. However, our understanding of the pathogenesis of TD is still lacking11.
Several hypotheses have been proposed to explain the development of TD, including dopamine receptor hypersensitivity, γ-aminobutyric acid (GABA) deficiency, and neuronal damage due to free radicals12,13. Recurring dyskinetic movements of facial and limb muscles, indistinguishable in form from tardive dyskinesia, are observed in patients with schizophrenia who have never been exposed to antipsychotics, suggesting that the intrinsic pathophysiology of schizophrenia contributes to tardive dyskinesia4. The leading hypothesis proposes that, as a result of chronic blocking of dopamine receptors, long-term antipsychotic treatments cause compensatory increase in dopamine receptor sensitivity14. On the other hand, TD symptoms may persist even after drug withdrawal, raising the issue that heightened dopamine receptor sensitivity may not account for all of the symptoms of TD.
One alternative hypothesis is that TD may be associated with structural brain changes15. Voxel-based morphometry (VBM) is a convenient and well-validated quantitative assessment of gray matter volumes (GMVs) by magnetic resonance imaging (MRI), frequently used to document changes in brain volumes in various neurological disorders16. Although not without limitations, VBM allows for an objective and automatic assessment of gray matter (GM) morphology. Few studies have focused on documenting MRI findings specific to TD, and their results were surprisingly inconsistent17–21. For example, Li et al.19 found that schizophrenics with TD had significantly reduced GMVs in the bilateral inferior and right superior frontal gyri in correlation with symptom severity. Sarro et al.20, however, found that TD-related volume reductions were predominantly subcortical, involving the basal ganglia and thalamus. The inconsistencies do not merely concern the anatomical locations of morphological changes; the putative correlations between morphology and TD symptom severity varied widely in the above-mentioned studies. These inconsistencies may in part be related to small sample sizes, heterogeneity in patient samples, or differences in imaging and analysis methods across the studies.
Here, we attempted to address morphological changes in TD with a more homogeneous Chinese patient sample, high-resolution imaging protocol, and standard VBM analytics. We hypothesized that TD-specific changes in GMVs would be identified in patients with schizophrenia and TD, and that the morphological changes would correlate with the clinical symptoms or severity of dyskinesia.
Results
Demographic and clinical characteristics
No significant differences in age, sex composition, and education were found among the three groups. Duration of illness and antipsychotic dose (CPZ) were indistinguishable between the TD and non-TD groups (all p’s > 0.05). However, the TD group had significantly higher PANSS Negative scores (p = 0.002) than the non-TD group (Table 1). No other PANSS scores were significantly different between the TD and non-TD groups.
Table 1.
Demographic and clinical characteristics.
| Variables | TD (n = 32) | Non-TD (n = 31) | HC (n = 32) | F/T/X 2 | P |
|---|---|---|---|---|---|
| Sex (M/F) | 20/12 | 19/12 | 19/13 | 4, 697 | 0.096 |
| Age (years) | 46.9 ± 10 | 45.7 ± 7.5 | 45.6 ± 7.7 | 0.174 | 0.841 |
| Education(years) | 11.5 ± 2.4 | 12.4 ± 2.2 | 11.9 ± 2.2 | 1.268 | 0.286 |
| Duration of illness(years) | 22.8 ± 10.2 | 22.6 ± 8.4 | — | 0.071 | 0.943 |
| PANSS | |||||
| Positive subscore | 14.4 ± 5.5 | 13.8 ± 5.6 | — | 0.405 | 0.687 |
| Negative subscore | 22.3 ± 5.8 | 17.3/6.2 | — | 3.311 | 0.002 |
| General psychopathological subscore | 30.9 ± 5.6 | 31.2 ± 7.2 | — | −0.157 | 0.876 |
| Total score | 67.6 ± 11.7 | 62.3 ± 13.8 | — | 1.657 | 0.103 |
| AIMS | 8.6 ± 4.2 | — | — | — | |
| CPZ equivalents (mg) | 508 ± 266 | 530 ± 224 | — | −0.35 | 0.727 |
| Haloperidol | 1 | 0 | |||
| Amisulpride | 1 | 0 | |||
| Clozapine | 16 | 10 | |||
| Risperidone | 4 | 6 | |||
| Olanzapine | 8 | 8 | |||
| Quetiapine | 2 | 0 | |||
| Perphenazine | 0 | 2 | |||
| Pipotiazine | 0 | 1 | |||
| Sulpiride | 0 | 1 | |||
| Aripiprazole | 0 | 2 | |||
| Lurasidone | 0 | 1 | |||
p-value indicate significant differences (p < 0.05) in appropriate statistical tests. PANSS: positive and negative syndrome scale; AIMS: Abnormal Involuntary Movement Scale; CPZ: chlorpromazine; TD: patients with schizophrenia and tardive dyskinesia; non-TD: patients with schizophrenia and without tardive dyskinesia; HC healthy subjects.
Voxel-based morphometry
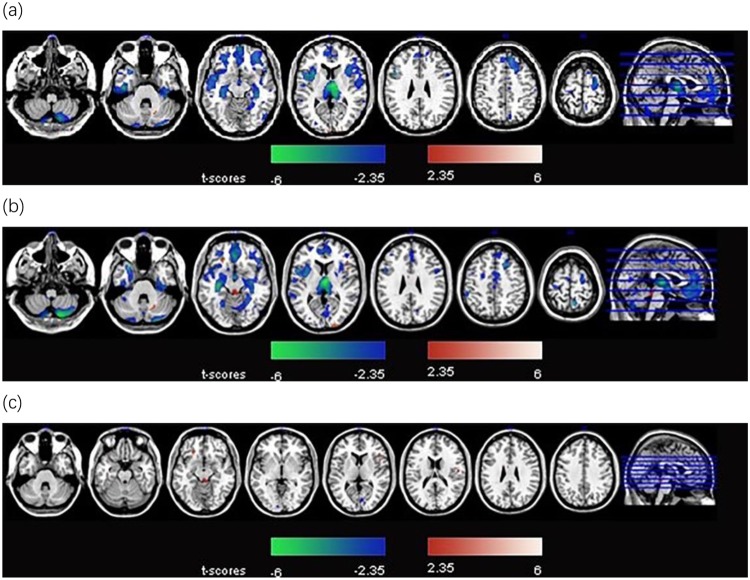
Schizophrenics both with and without TD showed significant GMV loss in multiple cortical areas including frontal and temporal cortices, insula and cerebellum, as compared with controls (p < 0.01). Further, the TD group demonstrated significantly greater volume reductions in the frontal areas than the non-TD group (p < 0.01) (Fig. 1a and b). The brainstem, inferior frontal gyrus and precentral gyrus demonstrated significant GMV increases in the TD group compared with the non-TD group; in contrast, the cuneus and lingual gyrus demonstrated significant GMV loss in the TD group relative to the non-TD group (Table 2 and Fig. 1c).
Figure 1.
(a) Regions showing gray matter volume changes in schizophrenics without tardive dyskinesia (non-TD), as compared with healthy control group. Warm and cool color each represents volume increases (non-TD > control) and decreases (non-TD < control). (b) Regions showing gray matter volume changes in schizophrenics with tardive dyskinesia group compared with healthy control group. Warm and cool color each represents volume increases (TD > control) and decreases (TD < control). (c) Regions demonstrating gray matter volume changes in schizophrenics with tardive dyskinesia as compared to those without tardive dyskinesia group. Warm and cool color each represents volume increases (TD > non-TD) and decreases (TD < non-TD).
Table 2.
Brain regions with significant volume variation in schizophrenics with tardive dyskinesia group compared to schizophrenics without tardive dyskinesia group.
| Voxels size Anatomical Region | X | Y | Z | BA | Cluster size | T-statistic |
|---|---|---|---|---|---|---|
| Brain stem | 0 | −35 | −21 | — | 204 | 3.33 |
| Inferior Frontal Gyrus | −18 | 30 | −14 | 11, 47 | 218 | 4.17 |
| Cuneus, Lingual Gyrus | 9 | −77 | 5 | 17, 18, 23, 30 | 182 | −3.22 |
| Precentral Gyrus | 54 | −12 | 15 | 43, 13 | 101 | 3.1 |
X, Y, Z: MNI (Montreal Neurological Institute) coordinates; BA: Brodmann area; p < 0.05 with extent threshold of 100 voxels; T-statistic: peak T score of the cluster.
Correlations between regional GMV changes and clinical evaluations of schizophrenia with/without TD
Among the regions that showed significant differences between the TD and non-TD groups, the cuneus and lingual gyrus changes in GMV were positively correlated with AIMS scores (r = 0.60, p = 0.001) in the TD group (Table 3 and Fig. 2), which was significant after Bonferroni correction for 4 regions of interest we tested in linear regression (p < 0.004). (Brodmann areas: 17, 18, 23, and 30).
Table 3.
Relationship between clinical evaluations and gray matter volume changes derived from the comparison of schizophrenics with and without tardive dyskinesia.
| Brain regions | PANSS P | PANSS N | PANSS G | PANSS T | AIMS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R-value | P-value | R-value | P-value | R-value | P-value | R-value | P-value | R-value | P-value | |
| Brainstem | 0.091 | 0.491 | 0.260 | 0.047 | −0.017 | 0.899 | 0.158 | 0.233 | 0.08 | 0.687 |
| Inferior Frontal Gyrus | −0.047 | 0.725 | 0.106 | 0.424 | −0.081 | 0.544 | −0.006 | 0.964 | 0.002 | 0.994 |
| Cuneus, Lingual Gyrus | −0.015 | 0.912 | −0.083 | 0.531 | −0.120 | 0.365 | −0.104 | 0.434 | 0.599 | 0.001 |
| Precentral Gyrus | −0.071 | 0.595 | 0.033 | 0.803 | −0.081 | 0.540 | −0.052 | 0.695 | 0.033 | 0.869 |
PANSS: positive and negative syndrome scale; AIMS, Abnormal Involuntary Movement Scale; BA: Brodmann Area; p-value indicate significant differences (p < 0.05) in appropriate statistical tests.
Figure 2.

The relationship between Abnormal Involuntary Movement Scale score and gray matter changes in the cuneus or lingual gyrus in the schizophrenics with tardive dyskinesia group. The unit is cm3.
Discussion
In this study, we found that, compared with the non-TD group, the TD group had increased GMVs in the brainstem, inferior frontal gyrus, and precentral gyrus, and reduced volume in the cuneus and lingual gyrus. Furthermore, the GMV changes in the latter areas were positively correlated with AIMS scores in the TD group. We also found that patients with schizophrenia had significantly reduced GMVs in multiple cortical areas regardless of the presence of TD.
Increased GMVs in the brainstem, inferior frontal gyrus, and precentral gyrus in TD relative to non-TD may relate to the cortico-striatal-thalamic loop of information processing. Cortical motor information is relayed to the basal ganglia, which in turn sends projections back via the thalamus to the cortex. The basal ganglia output nuclei, the substantia nigra pars reticulata, and the globus pallidus all project to the prefrontal cortex via specific thalamic nuclei22. Thus, information from the enlarged cortical gyri is processed in the basal ganglia, which project back to the cortex via projections to the motor thalamus. A major neurotransmitter of the cortico-striatal-thalamo-cortical circuits is GABA. GABAergic interneurons are normally responsible for maintaining a balance in functions between the direct and indirect cortico-striatal-thalamic-cortical pathways23. We hypothesize that a decrease in GABA levels results in attenuated inhibition of thalamic neurons, which leads to cortical over activity and increased cortical input, followed by compensatory cortical hyperplasia.
Previous studies showed that decreased GABA levels are associated with TD, and an indirect GABA agonist has demonstrated therapeutic efficacy for TD14. Masseter motor neurons are innervated bilaterally by premotor neurons extensively distributed in the brainstem24,25. The corticobulbar tract mainly terminates in the premotor neuron pool of the brainstem, which receives glutamatergic afferents from the cortex26. It is possible that the impaired movement in schizophrenics with TD may reflect the need of more cognitive resources to perform complex activities27. The increased GMV may reflect a consequence of chronic TD, a compensatory process of the cortico-striatal pallidal thalamocortical circuit in response to prolonged TD symptoms.
We also found that, compared with the non-TD group, the TD group showed decreased GMV in the cuneus and lingual gyrus. Further, in TD group, cuneus and lingual gyrus GMV was in positive correlation with patients’ AIMS scores. These findings were in contrast with previous reports of decreased GMV of cuneus and lingual gyrus in schizophrenia patients as compared to healthy participants28 and a positive correlation of more severe GMV decreases in the right superior frontal gyrus with AIMS score. It is very difficult to explain these contrasting and paradoxical findings. Further, previous studies have reported these frontal and visual cortical abnormality as a characteristic of schizophrenia29. Thus, the current findings remain to be confirmed and whether these structural brain changes are specific to TD remains to be investigated.
It is also worth noting that the cases included in Gupta’s mega-analysis29 were mostly medicated. Typical antipsychotics have been reported to be associated with enlarged GMV in schizophrenic patients30. However, the atypical antipsychotic clozapine was associated with a decrease in caudate size in schizophrenia patients previously treated with typical antipsychotics17. Here, the medication status of the TD and non-TD groups are indistinguishable. Therefore, the current GMV findings may not be due to medication treatment. In the current study TD and non-TD patients were indistinguishable in their medication treatments.
The leading models of the etiology of TD include dopamine receptor supersensitivity, GABA depletion, cholinergic deficiency, neurotoxicity, oxidative stress, changes in synaptic plasticity, and defective neuro-adaptive signaling. Other studies have suggested that TD may be related to reduction in GM volumes19,20, which would be consistent with our general observations herein. Previous studies described decreased GMV in the basal ganglia in TD20, which we failed to observe in the current study. There are a few potential explanations for this discrepancy. Firstly, the sample sizes of these two studies are moderate. Inconsistencies between small and moderate sized studies are not surprising in light of the wide diversity in the findings regarding GM abnormalities reported by small and moderate sized studies. Secondly, this diversity is probably largely due to the heterogeneity of the condition across subjects. The subjects in the study by Sarro et al. are Spanish, while those subjects in our study are Chinese. Thirdly, the methods of analyzing images are different. The data of the study by Sarro et al. were analyzed with FSL-VBM, while our study used VBM based on MATLAB. More studies with larger sample sizes are definitely warranted to resolve these discrepancies. Furthermore, severity of TD is associated with the severity of negative symptoms in schizophrenia. It is plausible that both dopamine blocking medication and the pathophysiology of schizophrenia itself contribute to TD4.
Several limitations should be considered when interpreting the results of the present study. The data are cross-sectional; therefore, the dynamic changes in brain structure during the progression of TD could not be assessed. The small sample size is another limitation of this study. A meta-analysis integrating multiple studies to generate a more comprehensive understanding of TD and grey matter variations is warranted. In particular, longitudinal studies would provide deeper insight into the relationship of cerebral GMV and the pathophysiology of TD. Finally, changes in functional activity and connectivity should be assessed along with cerebral morphometry to better understand the neural bases of TD.
In sum, although the availability of second generation antipsychotic medications has reduced the incidence and severity of TD, it remains a potentially severe side effect of the treatment for schizophrenia. The current results suggest that TD may be associated with the alterations in GMV that are different from that of schizophrenics without TD. Further studies are needed to validate this finding. These findings may facilitate new studies on the neural markers of this debilitating condition.
Methods
Participants
Thirty-two (20 men and 12 women, age: 46.9 ± 10.0 years) right-handed schizophrenics with TD (TD group), thirty-one schizophrenics (19 men and 12 women, age: 45.7 ± 7.3 years) without TD (non-TD group), and thirty-two (19 men and 13 women, age: 48.8 ± 7.9 years) healthy controls (HC group) were recruited for this study (Table 1). All patients were recruited from the inpatient services at Beijing HuiLongGuan Hospital. All schizophrenia patients met the following inclusion criteria: 1) age 18–60 years; 2) meeting the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) (DSM-IV-TR) criteria for schizophrenia after a structured clinical interview for DSM-IV-TR Axis I Disorders, Patient’s Version; 3) diagnosis with schizophrenia for at least 5 years; 4) having received stable doses of antipsychotics for at least 6 months before the study. The HC group comprised healthy residents drawn from the same period. Subjects were excluded if they met one of the following criteria: 1) a DSM-IV Axis I diagnosis other than schizophrenia; 2) serious, acute, unstable and/or significant and untreated medical illness (e.g. infection, diabetes, and uncontrolled hypertension); 3) a history of brain trauma or neurological disease; 4) pregnant or breast-feeding; 5) taken neurotrophic or free radical metabolism drugs within the 12 weeks prior to participation.
TD was diagnosed using Schooler and Kane’s Research Diagnostic Criteria (1982)31, and its severity was assessed using the Abnormal Involuntary Movements Scale (AIMS)32. The TD clinical ratings were performed by two experienced psychiatrists who were clinically trained to consistently perform AIMS testing as part of the general hospital training procedures, although they were not specifically trained for this study. In addition, patients with TD were re-evaluated for AIMS score by the same psychiatrist at least one month later, and were diagnosed with TD only if both evaluations yielded positive results. Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS)33. Both scales had intra class correlation coefficients ≥0.75. This study was approved by the Ethics Review Board (IRB) of the Beijing HuiLongGuan hospital. All participants gave written informed consent before participating in the study. All patients were recruited from the inpatient services at Beijing HuiLongGuan Hospital according to a cross-sectional naturalistic design. All study procedures followed this research protocol. We confirmed that all research was performed in accordance with the relevant guidelines.
Data acquisition
Structural MRI data were acquired on a 3.0-Tesla MR scanner (Trio system; Siemens, Erlangen, Germany) using a T1-weighted (T1W) sagittal 3D-MPRAGE (magnetization prepared rapid acquisition with gradient echo) sequence: echo time (TE) = 2.6 ms; inversion time (TI) = 800 ms; repetition time (TR) = 1,600 ms; flip angle (FA) = 9°; field of view (FOV) = 256 mm × 224 mm; matrix size = 512 × 448; slice thickness = 1 mm; voxel dimension = 1 mm × 1 mm × 1 mm. T2-weighted images (T2WI) were acquired using a turbo-spin-echo (TSE) sequence: 20 axial slices, thickness/gap = 5/1.5 mm, matrix = 512 × 416, TR = 4,140 ms, TE = 92 ms, FA = 150°, FOV = 187 mm × 230 mm.
VBM analysis
Individual high-resolution T1-weighted data were analyzed using the VBM8-Toolbox (http://dbm.neuro.uni-jena.de/vbm) technique34, implemented with Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8), and executed in MATLAB 2010 (https://www.mathworks.com/products/matlab.html). First, data from each participant were carefully checked three times for any scanner artifacts, motion problems or gross anatomical abnormalities. Then a noise reduction procedure was performed on each participant’s native space T1W structural image using a spatial-adaptive non-local-means denoising filter.
For image preprocessing, T1 images were first segmented into GM, white matter (WM) and cerebrospinal fluid (CSF) and normalized to an Montreal Neurological Institute (MNI) template using the unified standard segmentation option in SPM835. The total GMV of each voxel was obtained through modulation by multiplying the GM concentration map by the non-linear determinants derived from the spatial normalization step. All normalized, segmented, and modulated MNI standard space MR images were then visually checked for quality and smoothed using a Gaussian filter with a full-width at half-maximum smoothing kernel of 8 mm. Total intracranial volume (TIV) was calculated as the sum of GM, WM and CSF volumes. Finally, after image smoothing, the GM images were entered into statistical modeling to obtain cluster sizes for each group.
Statistical analysis
ANOVAs were used to compare the age and education among the three groups. Non-parametric tests were used to compare the sex composition among the three groups. T-tests were used to compare the PANSS scores (Total, Negative, Positive, and General), medication in chlorpromazine (CPZ) equivalents, and duration of illness between the patient groups. Voxel-wise GMV differences among the three groups were investigated using ANCOVA, with age, sex, education and TIV as covariates. To avoid possible edge effects around the margin between different tissue types, all voxels with a GM probability value lower than 0.2 were excluded. Statistical inferences were made with a voxel-level statistical threshold (p < 0.01), after applying an extent threshold (p < 0.001 uncorrected) with a minimum cluster size of 100 voxels (with voxel size equal to 1 mm3)36,37. Then we performed correlation analyses to explore the relationship between regions with nominal TD vs. non-TD differences, and that between GMV differences and severity of TD symptoms as assessed by AIMS in the TD group, again using age, sex, education and CPZ as covariates. Volume size, clinical symptoms (PANSS Total), and other clinical parameters were also explored. GMV group comparisons were performed in SPM8 and the correlation analyses between volume size of brain regions and AIMS were performed using SPSS 20.0 (IBM, USA).
Acknowledgements
This work was supported by National Natural Science Foundation of China (81071086, 81461130016), Beijing Natural Science Foundation (7151005), and by Capital Clinical Research Foundation (Z121107001012035). Our deepest gratitude goes to the anonymous reviewers for their thoughtful suggestions that have helped improve this paper substantially.
Author Contributions
Ting Yu, M.D. and Yanli Li, M.D. enrolled the subjects, performed the clinical ratings, wrote the protocol, conducted the analysis, and wrote the manuscript. Dr Fengmei Fan: conducted the MRI procedures for all subjects and analyzed imaging data. Dr. Hongbao Cao, Dr. Yin Yao Shugart, and L. Elliot Hong, M.D.: provided critical comments for the study design. Dr. Shuping Tan and Dr. Fude Yang: performed literature survey and provided conceptualized the study design. Dr. Xingguang Luo and Dr. Xiangyang Zhang: conceptualized the study design. Dr. Chiang-Shan R. Li: contributed to revision of the manuscript. Dr. Yunlong Tan: coordinated the clinical research team, provided critical comments for the study design and writing of the manuscript. All authors contributed to and have approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ting Yu and Yanli Li contributed equally.
References
- 1.Tian L, et al. Reduced Serum TNF Alpha Level in Chronic Schizophrenia Patients with Or without Tardive Dyskinesia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:259–264. doi: 10.1016/j.pnpbp.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Gopal S, et al. Incidence of Tardive Dyskinesia: A Comparison of Long-Acting Injectable and Oral Paliperidone Clinical Trial Databases. Int J Clin Pract. 2014;68:1514–1522. doi: 10.1111/ijcp.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An HM, et al. Extract of Ginkgo Biloba is Equivalent to Vitamin E in Attenuating and Preventing Vacuous Chewing Movements in a Rat Model of Tardive Dyskinesia. Behav Pharmacol. 2013;24:610–616. doi: 10.1097/FBP.0b013e3283656d87. [DOI] [PubMed] [Google Scholar]
- 4.Liddle PF. Tardive Dyskinesia in Schizophrenia. Br J Psychiatry. 2013;203:6–7. doi: 10.1192/bjp.bp.112.123679. [DOI] [PubMed] [Google Scholar]
- 5.Vinuela A, Kang UJ. Reversibility of Tardive Dyskinesia Syndrome. Tremor Other Hyperkinet Mov (N Y). 2014;4:282. doi: 10.7916/D86Q1VXZ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moilanen J, et al. Brain Morphometry of Individuals with Schizophrenia with and without Antipsychotic Medication - the Northern Finland Birth Cohort 1966 Study. Eur Psychiatry. 2015;30:598–605. doi: 10.1016/j.eurpsy.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Slovenko R. Update On Legal Issues Associated with Tardive Dyskinesia. J Clin Psychiatry. 2000;61(Suppl 4):45–57. [PubMed] [Google Scholar]
- 8.Chang FC, Fung VS. Clinical Significance of Pharmacogenomic Studies in Tardive Dyskinesia Associated with Patients with Psychiatric Disorders. Pharmgenomics Pers Med. 2014;7:317–328. doi: 10.2147/PGPM.S52806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pena MS, Yaltho TC, Jankovic J. Tardive Dyskinesia and Other Movement Disorders Secondary to Aripiprazole. Mov Disord. 2011;26:147–152. doi: 10.1002/mds.23402. [DOI] [PubMed] [Google Scholar]
- 10.Bai YM, et al. White Matter Abnormalities in Schizophrenia Patients with Tardive Dyskinesia: A Diffusion Tensor Image Study. Schizophr Res. 2009;109:167–181. doi: 10.1016/j.schres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Chouinard G. Interrelations Between Psychiatric Symptoms and Drug-Induced Movement Disorder. J Psychiatry Neurosci. 2006;31:177–180. [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoudi S, Levesque D, Blanchet PJ. Upregulation of Dopamine D3, Not D2, Receptors Correlates with Tardive Dyskinesia in a Primate Model. Mov Disord. 2014;29:1125–1133. doi: 10.1002/mds.25909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreassen OA, Jorgensen HA. Neurotoxicity Associated with Neuroleptic-Induced Oral Dyskinesias in Rats. Implications for Tardive Dyskinesia? Prog Neurobiol. 2000;61:525–541. doi: 10.1016/S0301-0082(99)00064-7. [DOI] [PubMed] [Google Scholar]
- 14.Rana AQ, Chaudry ZM, Blanchet PJ. New and Emerging Treatments for Symptomatic Tardive Dyskinesia. Drug Des Devel Ther. 2013;7:1329–1340. doi: 10.2147/DDDT.S32328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajagopalan V, Pioro EP. Disparate Voxel Based Morphometry (VBM) Results Between SPM and FSL Softwares in ALS Patients with Frontotemporal Dementia: Which VBM Results to Consider? Bmc Neurol. 2015;15:32. doi: 10.1186/s12883-015-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lui S, et al. Association of Cerebral Deficits with Clinical Symptoms in Antipsychotic-Naive First-Episode Schizophrenia: An Optimized Voxel-Based Morphometry and Resting State Functional Connectivity Study. Am J Psychiatry. 2009;166:196–205. doi: 10.1176/appi.ajp.2008.08020183. [DOI] [PubMed] [Google Scholar]
- 17.Scheepers FE, et al. The Effect of Clozapine On Caudate Nucleus Volume in Schizophrenic Patients Previously Treated with Typical Antipsychotics. Neuropsychopharmacol. 2001;24:47–54. doi: 10.1016/S0893-133X(00)00172-X. [DOI] [PubMed] [Google Scholar]
- 18.Honea RA, et al. Is Gray Matter Volume an Intermediate Phenotype for Schizophrenia? A Voxel-Based Morphometry Study of Patients with Schizophrenia and their Healthy Siblings. Biol Psychiatry. 2008;63:465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li CT, et al. Gray Matter Abnormalities in Schizophrenia Patients with Tardive Dyskinesia: A Magnetic Resonance Imaging Voxel-Based Morphometry Study. Plos One. 2013;8:e71034. doi: 10.1371/journal.pone.0071034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarro S, et al. Structural Brain Changes Associated with Tardive Dyskinesia in Schizophrenia. Br J Psychiatry. 2013;203:51–57. doi: 10.1192/bjp.bp.112.114538. [DOI] [PubMed] [Google Scholar]
- 21.Telfer S, Shivashankar S, Krishnadas R, McCreadie RG, Kirkpatrick B. Tardive Dyskinesia and Deficit Schizophrenia. Acta Psychiatr Scand. 2011;124:357–362. doi: 10.1111/j.1600-0447.2011.01751.x. [DOI] [PubMed] [Google Scholar]
- 22.Mushiake H, Strick PL. Pallidal Neuron Activity During Sequential Arm Movements. J Neurophysiol. 1995;74:2754–2758. doi: 10.1152/jn.1995.74.6.2754. [DOI] [PubMed] [Google Scholar]
- 23.Teo JT, Edwards MJ, Bhatia K. Tardive Dyskinesia is Caused by Maladaptive Synaptic Plasticity: A Hypothesis. Mov Disord. 2012;27:1205–1215. doi: 10.1002/mds.25107. [DOI] [PubMed] [Google Scholar]
- 24.Jian C, et al. Multiparameter Electromyography Analysis of the Masticatory Muscle Activities in Patients with Brainstem Stroke at Different Head Positions. Front Neurol. 2017;8:221. doi: 10.3389/fneur.2017.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nozaki S, Iriki A, Nakamura Y. Localization of Central Rhythm Generator Involved in Cortically Induced Rhythmical Masticatory Jaw-Opening Movement in the Guinea Pig. J Neurophysiol. 1986;55:806–825. doi: 10.1152/jn.1986.55.4.806. [DOI] [PubMed] [Google Scholar]
- 26.Nozaki S, Iriki A, Nakamura Y. Trigeminal Premotor Neurons in the Bulbar Parvocellular Reticular Formation Participating in Induction of Rhythmical Activity of Trigeminal Motoneurons by Repetitive Stimulation of the Cerebral Cortex in the Guinea Pig. J Neurophysiol. 1993;69:595–608. doi: 10.1152/jn.1993.69.2.595. [DOI] [PubMed] [Google Scholar]
- 27.Zeuner KE, et al. Increased Volume and Impaired Function: The Role of the Basal Ganglia in Writer’s Cramp. Brain Behav. 2015;5:e301. doi: 10.1002/brb3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mane A, et al. Progressive Gray Matter Changes in First Episode Schizophrenia: A 4-Year Longitudinal Magnetic Resonance Study Using VBM. Schizophr Res. 2009;114:136–143. doi: 10.1016/j.schres.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Gupta CN, et al. Patterns of Gray Matter Abnormalities in Schizophrenia Based on an International Mega-Analysis. Schizophrenia Bull. 2015;41:1133–1142. doi: 10.1093/schbul/sbu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakos MH, et al. Increase in Caudate Nuclei Volumes of First-Episode Schizophrenic Patients Taking AntipsychoticDrugs. AM J Psychiat. 1994;151:1430. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- 31.Schooler NR, Kane JM. Research Diagnoses for Tardive Dyskinesia. Arch Gen Psychiatry. 1982;39:486–487. doi: 10.1001/archpsyc.1982.04290040080014. [DOI] [PubMed] [Google Scholar]
- 32.Burti L, Parolin A, Zanotelli R. [Tardive Dyskinesia. AIMS (Abnormal Involuntary Movement Scale) as a Diagnostic and Research Tool] Minerva Med. 1981;72:2829–2836. [PubMed] [Google Scholar]
- 33.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 34.Ashburner J, Friston KJ. Voxel-Based Morphometry–The Methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 35.Tzourio-Mazoyer N, et al. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 36.Guo W, et al. Decreased Gray Matter Volume in the Left Middle Temporal Gyrus as a Candidate Biomarker for Schizophrenia: A Study of Drug Naive, First-Episode Schizophrenia Patients and Unaffected Siblings. Schizophr Res. 2014;159:43–50. doi: 10.1016/j.schres.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 37.Fan F, et al. Dynamic Brain Structural Changes After Left Hemisphere Subcortical Stroke. Hum Brain Mapp. 2013;34:1872–1881. doi: 10.1002/hbm.22034. [DOI] [PMC free article] [PubMed] [Google Scholar]