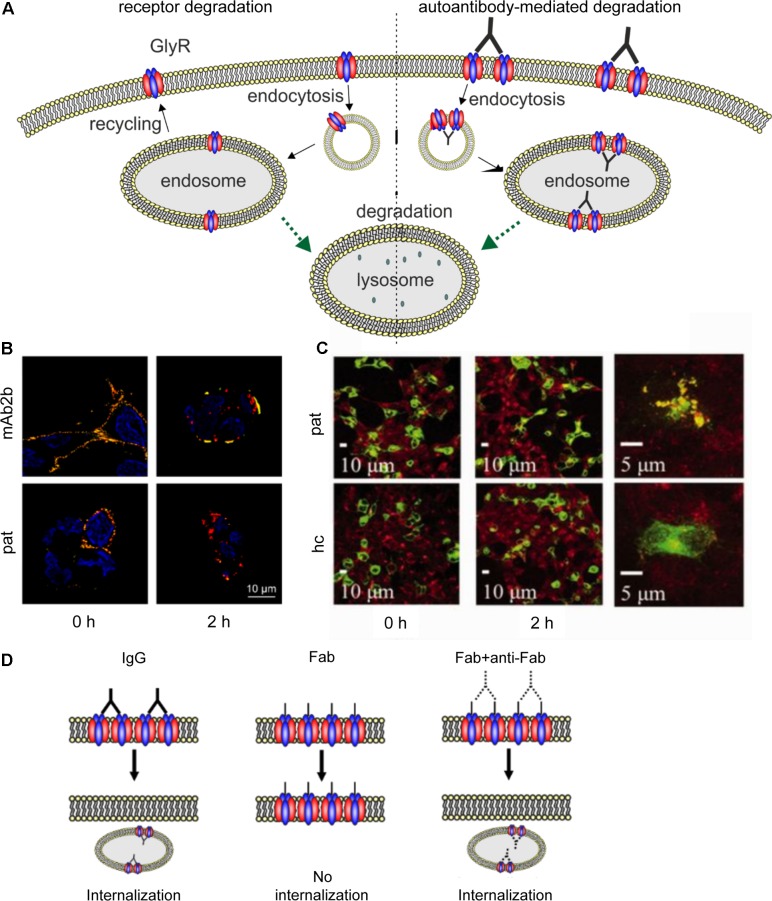
FIGURE 5.
Native GlyR internalization compared to autoantibody-mediated GlyR internalization. (A) Under native conditions, ubiquitination of cell surface GlyRs initiates receptor degradation by endocytosis followed by lysosomal or lysosomal-like vacuolar degradation (left). Autoantibodies crosslink GlyRs, thus inducing internalization and degradation by endosomal and lysosomal pathways (right). (B) GlyR α1-transfected HEK293 cells were incubated with monoclonal GlyR α1-specific antibody mAb2b or patient serum (pat) and internalization was induced by incubation at 37°C for 0 or 2 h. Patient serum as well as mAb2b were able to induce internalization at 2 h. Red = internalized GlyRs; yellow = membrane-integrated GlyRs; blue = DAPI staining. (C) GlyR α1-EGFP expressing HEK293 cells were incubated with patient serum for 0 or 2 h at 37°C to induce internalization and stained with late endosomal marker LAMP2. Colocalization of both signals is higher in cells incubated with patient serum than with healthy control (hc). Green = GlyR α1-EGFP signal; red = LAMP2 signal (taken with permission from Carvajal-Gonzalez et al., 2014). (D) IgG binding to receptors is able to induce internalization (left), whereas Fab fragments alone cannot elicit receptor internalization (middle). Internalization can re-occur, when Fab fragments and anti-Fab antibodies are incubated together (right), indicating that crosslinking of receptors is required for internalization.