Abstract
Atmospheric concentration of carbon dioxide (CO2) is increasing at an unprecedented rate and subsequently leading to ocean acidification. Concomitantly, ocean warming is intensifying, leading to serious and predictable biological impairments over marine biota. Reef-building corals have proven to be very vulnerable to climate change, but little is known about the resilience of non-reef-building species. In this study, we investigated the effects of ocean warming and acidification on the antioxidant enzyme activity (CAT—catalase, and GST—glutathione S-transferase), lipid peroxidation (using malondialdehyde, MDA—levels as a biomarker) and heat shock response (HSP70/HSC70 content) of the octocoral Veretillum cynomorium. After 60 days of acclimation, no mortalities were registered in all treatments. Moreover, CAT and GST activities, as well as MDA levels, did not change significantly under warming and/or acidification. Heat shock response was significantly enhanced under warming, but high CO2 did not have a significant effect. Contrasting to many of their tropical coral-reef relatives, our findings suggest that temperate shallow-living octocorals may be able to physiologically withstand future conditions of increased temperature and acidification.
Electronic supplementary material
The online version of this article (10.1007/s12192-018-0919-9) contains supplementary material, which is available to authorized users.
Keywords: Climate change, Heat shock proteins, Antioxidant enzymes, Lipid peroxidation, Veretillum cynomorium
Introduction
Since the start of the industrial revolution, atmospheric concentrations of carbon dioxide (CO2) have been rising, reaching for the first time levels above 400 ppm (Lüthi et al. 2008) and being expected to reach 730–1020 ppm by the end of the century (Pörtner et al. 2014). Nearly 30% of atmospheric CO2 is absorbed by the oceans (Hoegh-Guldberg et al. 2014), resulting in a change in seawater chemistry that is expected to prompt a 0.13–0.42 pH unit drop in seawater pH by the year 2100 (Pörtner et al. 2014). Increasing atmospheric CO2 concentration is also increasing global temperature, with future projections pointing out to a rise up to 4 °C in sea surface temperature until the end of the century (Collins et al. 2013). Additionally, the frequency and extent of extreme weather events, such as heat waves, are expected to increase at a global scale (Pörtner et al. 2014). These changes are expected to cause major shifts in species distribution, phenology, and physiology (e.g., Edwards and Richardson 2004; Harvey et al. 2013; Kroeker et al. 2013; Parmesan and Yohe 2003), which can ultimately lead to worldwide extinction events (Thomas et al. 2004).
Important constituents of coral reef ecosystems in tropical regions and abundant components of shallow-water habitats in temperate environments, corals harbor an overwhelming complexity of species and play an essential ecological role within benthic communities (Carpenter et al. 2008; Freiwald et al. 2004). Considering their ecological relevance, large efforts have been undertaken in order to study the effects of climate change on corals worldwide (e.g., Baker et al. 2008; Hofmann et al. 2008). In fact, temperature and CO2 levels have been proven to affect several life-history processes of reef-building corals (e.g., development and reproduction), besides damaging several key physiological functions, such as calcification, photosynthesis, and respiration (Ateweberhan et al. 2013; Pandolfi et al. 2011). Moreover, increased seawater temperature is also threatening the symbiosis between corals and zooxanthellae, in a phenomenon known as coral bleaching (Glynn 1996). In contrast to reef-building corals, studies targeted to non-reef-building corals are still scarce. In fact, investigations addressing the effects of climate change on soft corals are mainly focused on community spatial distribution rather than in understanding the effects upon species ecophysiology (e.g., Gómez et al. 2015; Inoue et al. 2013; Ruzicka et al. 2013).
In order to avoid deleterious effects caused by environmental disturbances, marine organisms display a diverse set of physiological regulatory defense mechanisms, which include heat shock and antioxidant responses (Feder and Hofmann 1999; Lesser 2006). Heat shock response involves the synthesis of heat shock proteins (HSP), which possess an important role by promoting stabilization and refolding of denatured proteins (Dong et al. 2008; Tomanek 2010). On the other hand, antioxidant response is characterized by a powerful set of enzymatic antioxidants [e.g., catalase (CAT) and glutathione S-transferase (GST)], which act against toxic effects of reactive oxygen species [ROS, i.e., superoxide radicals (O2−), hydrogen peroxides (H2O2), singlet oxygens (1O2), and hydroxyl radicals (HO•)] (Apel and Hirt 2004). Under environmental stress, high levels of ROS production may cause cellular damage through lipid peroxidation, one of the most frequent cellular injury processes where ROS react with membrane-associated lipids (Lesser 2011).
Over the last few decades, the effects of increasing temperature and hypercapnia on the physiological defense mechanisms of corals have been extensively studied (see Supplementary Table SI). However, the bulk of these studies are focused on hexacorals species (e.g., Agostini et al. 2016; Downs et al. 2013; Downs et al. 2000; Griffin and Bhagooli 2004; Griffin et al. 2006), while studies on octocorals remain scarce (Madeira et al. 2015; Mydlarz and Jacobs 2006; Wiens et al. 2000). Moreover, most of the above-mentioned studies focus on the effects of temperature alone, without considering the effect of ocean acidification or the interaction between stressors.
The finger-shaped sea pen, Veretillum cynomorium (Pallas 1766), is a colonial octocoral belonging to the order Pennatulacea. This species is widely distributed in the eastern Atlantic Ocean (Vander Land 2008), and it is found on coastal shallow waters, inhabiting the soft sediment of beaches and sand plains (Cornelius et al. 1995), with a bathymetric distribution between 0 and circa 200 m (López-González et al. 2001). Previous studies have already addressed the great tolerance of this species to deal with the extreme abiotic conditions of the intertidal environment, as they are fitted with an anticipatory response to cope with oxidative stress during air exposure at low tide conditions (Teixeira et al. 2013). Additionally, it was also seen that they tolerate well the seasonal variation in temperature regimes, being able to withstand low tide conditions during summer without undergoing cellular damage (Madeira et al. 2015). Nevertheless, this species resilience toward climate change-related conditions remains unknown. To that end, the aim of the present study was to investigate the physiological mechanisms that may enable V. cynomorium to withstand future ocean warming and acidification conditions. More specifically, we analyzed (i) antioxidant enzyme activities (GST and CAT), (ii) lipid peroxidation (malondialdehyde—MDA), and (iii) heat shock response (HSP70/HSC70).
Materials and methods
Coral collection and laboratory acclimation
Twenty-four V. cynomorium colonies were hand-collected near the mouth of the Sado estuary (38° 29′ 11′′ N, 8° 53′ 13′′ W, Setúbal, Portugal) in March 2014. After field collection, organisms were immediately transported to the aquatic facilities of Laboratório Marítimo da Guia (Cascais, Portugal).
Octocorals were maintained in twelve 50-L holding tanks coupled to recirculation aquaculture systems filled with 0.35 μm-filtered (Harmsco, USA) and UV-irradiated (V2ecton 600, TMC Iberia, Portugal) natural seawater, directly pumped from the sea. Each system was fitted with mechanical (Glass wool, Fernando Ribeiro Lda, Portugal), biological (Ouriço®, Fernando Ribeiro Lda, Portugal), and physico-chemical filtration (V2Skim Pro 450, TMC Iberia, Portugal), with additional UV-irradiation (V2ecton 300, TMC Iberia, Portugal). A 10% seawater renewal was daily performed. Ammonia, nitrite, and nitrate levels were daily monitored by means of colorimetric tests (Profi Test, Salifert, Holland) and kept below detectable levels. During the experimental exposure, a photoperiod of 14 h:10 h (light:dark cycle) was performed. All colonies were fed (twice a day) with a mixture of frozen Artemia spp. and Mysis spp. (TMC Iberia, Portugal).
Upon arrival, colonies were acclimated for 15 days to the prevailing natural conditions of the collection site (temperature 19 ± 1 °C, pH 8.0 ± 0.1, pCO2~500 μatm, salinity 35 ± 1). Subsequently, organisms were exposed for 60 days to different experimental conditions (three tanks per treatment): (i) control temperature and normocapnia (19 °C, pH 8.0, pCO2~460 μatm); (ii) control temperature and hypercapnia (19 °C, pH 7.7, pCO2~1020 μatm); (iii) warming and normocapnia (26 °C, pH 8.0, pCO2~430 μatm); and (iv) warming and hypercapnia (26 °C, pH 7.7, pCO2~1060 μatm). The warming condition was chosen based on the temperature values observed during heat events in the collection site (see Supplementary Fig. S1). Regarding the pCO2 scenarios, one should keep in mind that this species inhabits the Western Iberian Upwelling Ecosystem, part of the Canary Current Upwelling System. In these regions, actual pCO2 levels may reach up to 500 μatm (Álvarez-Salgado et al. 1997; Perez et al. 1999) and are thus expected to exceed the level of 420–940 μatm projected for 2100 (Pörtner et al. 2014).
Water temperature and pH were continuously controlled and adjusted by means of an automatic monitoring device (Profilux 3.1, GHL, Germany), connected to temperature and pH probes (PL-0094 and PL-0071, respectively, GHL, Germany). Upon demand, seawater temperature was upregulated using submerged digital heaters (V2Therm 200 W, TMC Iberia, Portugal) or downregulated using seawater chillers (HC-250A, Hailea, China). Adjustment of pH levels was performed automatically via solenoid valves connected to the Profilux system. Reduction of pH values was accomplished by the injection of a certified CO2 gas (Airliquide, Portugal), while upregulation was achieved by the injection of CO2-filtered atmospheric air (using soda lime, Sigma-Aldrich, Portugal). Additionally, a daily monitoring of seawater temperature (thermometer TFX 430, WTW GmbH, Germany), salinity (V2 Refractometer, TMC Iberia, Portugal), and pH (pH/ion meter SG8, Mettler-Toledo, Switzerland) was performed using handheld equipment. Seawater carbonate system speciation (see Supplementary Table SII) was calculated weekly from total alkalinity (determined according to Sarazin et al. 1999) and pH measurements, using the CO2SYS software, with dissociation constants from Mehrbach et al. (1973) as refitted by Dickson and Millero (1987).
At the end of the experimental exposure, colonies were collected, immediately placed in liquid nitrogen, and stored at − 80 °C for subsequent biochemical analyses.
Biochemical analyses
Preparation of tissue extracts
Tissue samples from different colonies (n = 2 per tank, n = 6 per treatment) were individually homogenized in phosphate buffer saline (PBS), according to Lopes et al. (2013). Homogenates were centrifuged at 14,000xg for 20 min at 4 °C. Antioxidant enzyme activity, lipid peroxidation, and the heat shock response were quantified in the supernatant fraction. Each sample was run in triplicate (technical replicates) and results were normalized to total protein content, as described by Bradford (1976).
Antioxidant enzymes
CAT activity was determined following Aebi (1984). A total of 100 μL of each sample was added to 2.9 mL of substrate solution [50 mM potassium phosphate buffer (pH 7.0) and 12.1 mM H2O2], into quartz cuvettes. Absorbance was measured at 240 nm (Helios spectrophotometer, Unicam, UK), during 15-s intervals across a 180-s incubation period. Bovine CAT solution was used as a positive control to validate the assay. CAT activity was calculated based on the absorbance increase per minute, using the H2O2 extinction coefficient (0.04 ƐmM).
GST activity was determined according to Habig et al. (1974). A total of 180 μL of substrate solution [200 mM l-glutathione reduced, Dulbecco phosphate-buffered saline and 100 mM 1-chloro-2,4-dinitrobenzene (CDNB) solution] was added to 96-well microplates, alongside with 20 μL of GST standard or sample. Equine liver GST was used as a positive control to validate the assay. Enzyme activity was determined spectrophotometrically at 340 nm (Bio-Rad, Benchmark, USA), every minute during a 6-min time frame. GST activity was calculated based on the absorbance increase per minute, using the CDNB extinction coefficient (5.3 ƐmM).
Lipid peroxidation
Lipid peroxidation was determined by MDA quantification, according to the thiobarbituric acid reactive substances (TBARS) protocol (Uchiyama and Mihara 1978). Briefly, 10 μL of each sample was added to 50 mM of monobasic sodium phosphate buffer (45 μL), followed by the addition of 8.1% of sodium dodecyl sulphate (12.5 μL), 20% of trichloroacetic acid (93.5 μL), and 1% of thiobarbituric acid (93.5 μL). A volume of 50.5 μL of Milli-Q ultrapure water was added to this mixture, being subsequently mixed for 30 s and incubated in boiling water for 10 min. The resulting mixture was placed on ice for 3 min to lower temperature. Afterwards, 62.5 μL of Milli-Q ultrapure water and 312.5 μL of n-butanol pyridine (15:1 v/v) were added and microtubes centrifuged at 2000xg for 5 min. The supernatant fraction (150 μL) was added to 96-well microplates and absorbance was read at 532 nm (Bio-Rad, Benchmark, USA). MDA concentrations were calculated based on a calibration curve (0–0.3 μM) using MDA bis (dimethyl acetal) standards.
Heat shock response
The HSP70/HSC70 content was assessed through enzyme-linked immunosorbent assay (ELISA), according to an adaptation of the method described by Njemini et al. (2005). Each sample (10 μL) was diluted in 990 μL of PBS. Afterwards, 50 μL of each diluted sample was added to 96-well microplates (Microloan 600, Greiner, Germany) and incubated overnight at 4 °C. After 24 h, microplates were washed (four times) using PBS containing 0.05% Tween-20. Then, 100 μL of blocking solution (1% bovine serum albumin) was added to each well and microplates were incubated in darkness for 2 h at room temperature. Consequently, 50 μL of primary antibody HSP70/HSC70 (5 μg mL−1, Acris, USA) was added to each well. Microplates were incubated overnight at 4 °C and washed 24 h afterwards to remove non-linked antibodies. The alkaline phosphatase-conjugated anti-mouse IgG (Fab specific, Sigma-Aldrich, USA) was used as a secondary antibody, by adding 50 μL (1 μg mL−1) to each well, and microplates were incubated for 90 min at 37 °C. After an additional washing procedure, 100 μL of substrate p-nitrophenyl phosphate tablets were added to each well and incubated for 30 min at room temperature. Lastly, 50 μL of stop solution (3 M NaOH) were added to each well, with absorbance being read at 405 nm in a microplate reader (BIO-RAD, Benchmark, USA). Heat shock protein content was calculated from the calibration curve, based on serial dilutions (0–2000 μg mL−1) of purified HSP70 active protein (Acris, USA).
Statistical analyses
Generalized linear models (GLM) analysis was used to ascertain significant differences between temperature and pH treatments. For each dependent variable (CAT, GST, MDA, and HSP70/HSC70), temperature (2 levels: 19 and 26 °C) and pH (2 levels: 8.0 and 7.7) were used as explanatory variables, as well as their interaction. Mixed models were used to infer significant differences between replicate tanks within each treatment. Since there were no significant differences, the random effect of the tank was removed from the models. Our data was fitted using Gaussian family models. Model residuals were checked for departures from the assumed distributions and no significant deviations were found. Homogeneity and normality assumptions were checked through Levene and Shapiro tests, respectively. The most parsimonious models were selected based on the Akaike Information Criterion (Quinn and Keough 2002). Independence and leverage of the residuals were used to perform model validation. All statistical analyses were performed on R Studio (R Development Core Team 2016), using the lme4 and nlme packages.
Results
After 60 days of exposure to upcoming ocean warming and acidification conditions, no colony mortality was observed in all experimental treatments.
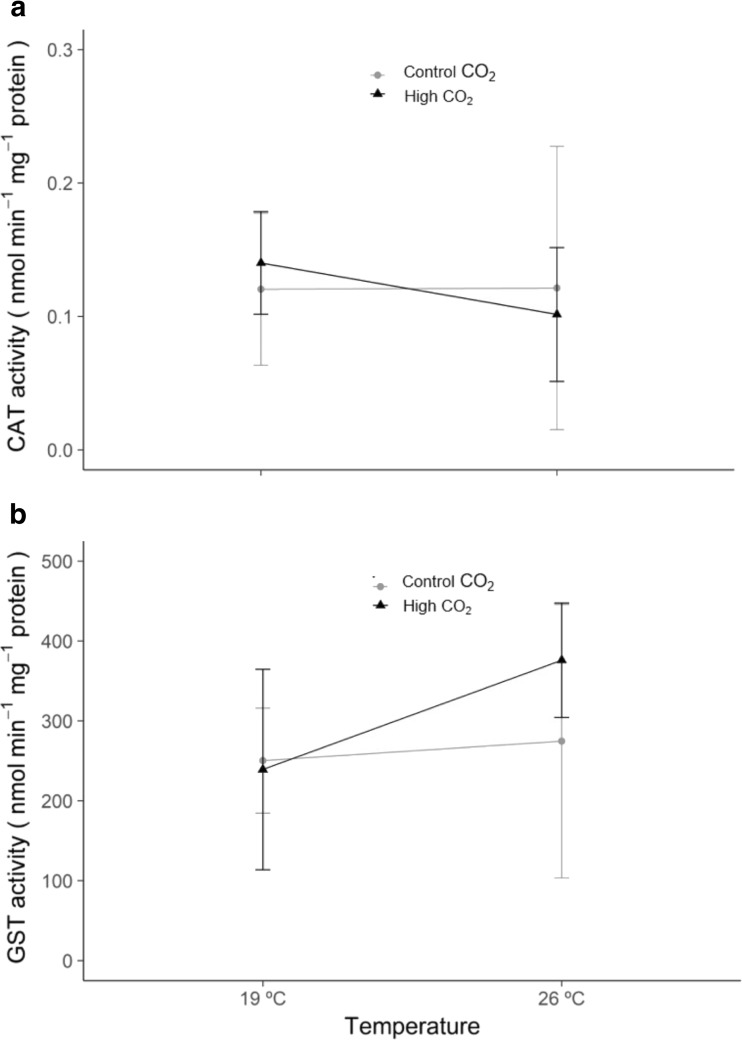
The activity of the antioxidant enzymes CAT and GST (Fig. 1) did not change significantly under warming and/or acidification (p > 0.05, GLM analysis in Supplementary Table SIII).
Fig. 1.
Antioxidant enzyme activities in Veretillum cynomorium under ocean warming and acidification conditions: a Catalase (CAT) and b glutathione S-transferase (GST). Values represent mean ± standard deviation
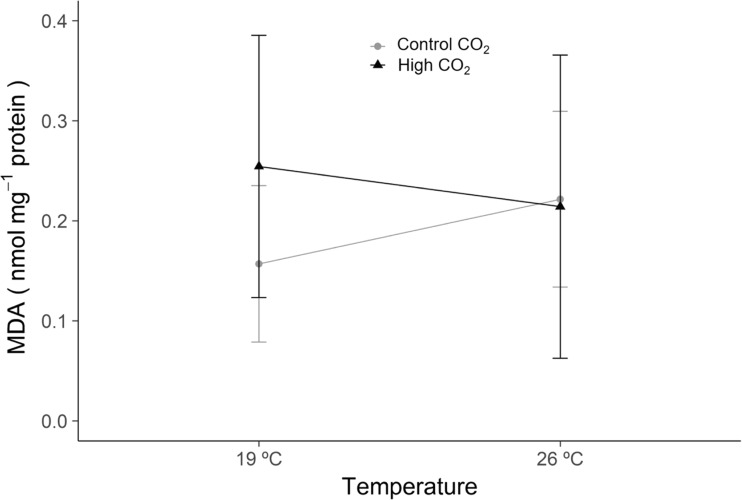
MDA levels (Fig. 2), a specific end-product of lipid peroxidation, also did not change significantly after exposure to ocean warming and/or acidification conditions (p > 0.05, GLM analysis in Supplementary Table SIII).
Fig. 2.
Lipid peroxidation (MDA—malondialdehyde) levels in Veretillum cynomorium under ocean warming and acidification conditions. Values represent mean ± standard deviation
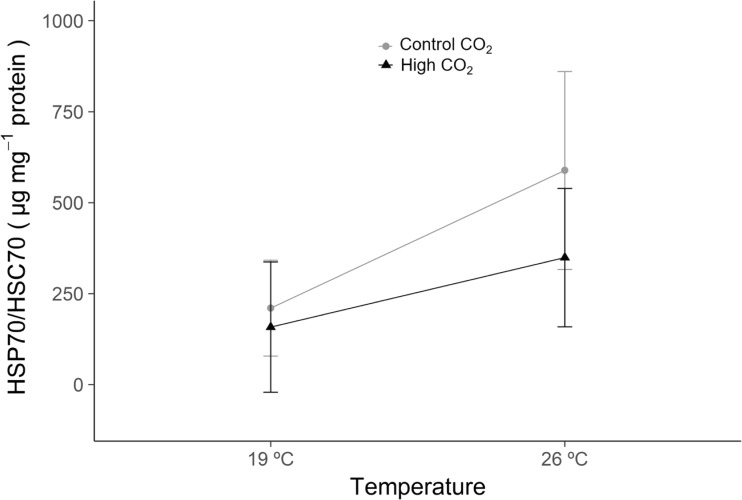
In contrast, the heat shock response (HSP70/HSC70, Fig. 3) was significantly enhanced under warming (p = 0.009, GLM analysis in Supplementary Table SIII), increasing from 210.1 ± 131.6 to 588.6 ± 271.9 μg mg−1 protein under normocapnia, and from 158.6 ± 179.1 to 349.0 ± 190.2 μg mg−1 protein under hypercapnia. Neither the effect of acidification nor the interaction between warming and acidification were significant (p > 0.05, GLM analysis in Supplementary Table SIII).
Fig. 3.
Heat shock protein (HSP70/HSC70) concentrations in Veretillum cynomorium under ocean warming and acidification conditions. Values represent mean ± standard deviation
Discussion
In the last decade, a significant body of research has been accumulated on the impact of climate change on coral ecophysiology, mainly on reef-building species (see Supplementary Table SI). Nonetheless, the impact of future ocean conditions on soft corals remains poorly known. To the best of our knowledge, only three studies were conducted so far in order to infer the impact of rising sea temperature on octocorals (Madeira et al. 2015; Mydlarz and Jacobs 2006; Wiens et al. 2000), and none of these studies have evaluated the effect of ocean acidification or the combination between increasing temperature and acidification.
Increased seawater temperature is known for enhancing ROS production and activating antioxidant enzymes, which are essential to eliminate ROS and prevent cellular damage such as lipid peroxidation (Lesser 2011). In most hard corals, the heat shock and antioxidant defense mechanisms are triggered under warming and/or acidified conditions (see Supplementary Table SI). However, in many cases, they are not able to avoid cellular damage in coral tissues (e.g., Downs et al. 2000; Flores-Ramírez and Liñán-Cabello 2007; Ritson-Williams et al. 2016; Soriano-Santiago et al. 2013; Yakovleva et al. 2009). Contrarily to reef-building corals, the present study showed that in the octocoral V. cynomorium, CAT and GST activities were not significantly affected by warming and/or acidification, and neither was cellular damage caused by lipid peroxidation. On the other hand, heat shock proteins, which play an important role in thermotolerance by helping denatured proteins to stabilize and refold (Tomanek 2010), were activated as a defense mechanism against high temperature, i.e., increasing significantly under warming conditions.
It is worth mentioning that the observed high tolerance of V. cynomorium to such abiotic conditions is not surprising, since this species can be found in coastal shallow habitats that are daily subject to extreme abiotic fluctuations (e.g., temperature, salinity, oxygen). Indeed, this species proved to tolerate rapid cyclical fluctuations of the intertidal environment, by presenting integrated heat shock and antioxidant responses that allow them to cope with the underlying oxidative stress to which they are frequently exposed during the emersion stage (Teixeira et al. 2013) and across thermal gradients (Madeira et al. 2015). In both studies, V. cynomorium proved to be equipped with powerful defense mechanisms that enable them to avoid peroxidative damage under stressful conditions. Thus, in a time when climate change threatens coral reefs all over the world, the octocoral V. cynomorium stands out for its great resilience to warming and acidification.
Comparative studies between symbiotic and non-symbiotic corals clearly showed that symbiotic corals are more vulnerable to high temperatures and are at greater risk (Baker et al. 2008; Pandolfi et al. 2011). This may be due to the fact that increased seawater temperature leads to coral bleaching and mortality, which is primarily initiated with an overproduction of ROS in the symbionts (Downs et al. 2002; Lesser 2006; Mydlarz et al. 2009), impairing the association between corals and zooxanthellae (Glynn 1996). In contrast, octocorals such as V. cynomorium, instead of an endosymbiotic algae assemblage, harbor microbial communities within their tissues (Baptista et al. 2012), which might confer broader tolerance to heat and chemical stress. In fact, recent studies have brought to light that the resistance of a coral is not only determined by the coral itself, but rather to the association between all its parts, i.e., coral, zooxanthellae, and associated microorganisms (Grottoli et al. 2018; Roche et al. 2018), and that a shift in coral symbiosis elements to specific microorganisms could eventually enhance their thermal resistance (Torda et al. 2017).
On the other hand, hard corals have also shown to be quite vulnerable to ocean acidification, since hypercapnia is reducing ocean carbonate ion availability and compromising the capacity of hard corals to build their skeletons (Carpenter et al. 2008). In contrast, V. cynomorium lacks an external calcium carbonate body. Instead, it presents a central axial skeleton composed by a fibrillar collagenous matrix calcified with calcite (Ledger and Franc 1978) and covered by an external tissue that may act as a barrier against decreased seawater pH, as previously observed in another octocoral species (Gabay et al. 2014).
In conclusion, the present study shows that exposure to ocean warming and acidification conditions did not have a negative impact on V. cynomorium physiology. Warmer conditions enhanced the heat shock response, a defense mechanism that allows them to tolerate higher temperatures, while the antioxidant response and cellular damage were not significantly affected. In contrast to reef-building corals that have shown to be particularly sensitive to climate-induced changes (Baker et al. 2008), the present findings show that V. cynomorium is a resilient species in the face of warming and acidified conditions and is expected to be able to withstand predicted future ocean conditions associated with climate change. Nevertheless, further studies are essential to evaluate the impact of future ocean scenarios on octocoral larvae, since the greater vulnerability of the early stages of development may become the bottleneck for species persistence in a changing ocean.
Electronic supplementary material
(DOCX 18.5 kb)
(DOCX 104 kb)
(DOCX 13.9 kb)
(DOCX 14.2 kb)
Acknowledgments
This project was supported by the Portuguese Foundation for Science and Technology (FCT), through financial support to MARE (UID/MAR/04292/2013), a doctoral grant to Ana Rita Lopes (SFRH/BD/97070/2013), postdoctoral grants to Filipa Faleiro (SFRH/BPD/79038/2011), Marta Pimentel (SFRH/BPD/117533/2016) and Tiago Repolho (SFRH/BPD/94523/2013), and an Investigador FCT consolidation grant to Rui Rosa.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aebi H. Catalase. In: Packer L, editor. Methods Enzymol. Orlando: Academic; 1984. pp. 121–126. [Google Scholar]
- Agostini S, Fujimura H, Hayashi H, Fujita K. Mitochondrial electron transport activity and metabolism of experimentally bleached hermatypic corals. J Exp Mar Biol Ecol. 2016;475:100–107. doi: 10.1016/j.jembe.2015.11.012. [DOI] [Google Scholar]
- Álvarez-Salgado XA, Castro CG, Pérez FF, Fraga F. Nutrient mineralisation patterns in shelf waters of the western Iberian upwelling. Cont Shelf Res. 1997;17:1247–1270. doi: 10.1016/S0278-4343(97)00014-9. [DOI] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Ateweberhan M, Feary DA, Keshavmurthy S, Chen A, Schleyer MH, Sheppard CR. Climate change impacts on coral reefs: synergies with local effects, possibilities for acclimation, and management implications. Mar Pollut Bull. 2013;74:526–539. doi: 10.1016/j.marpolbul.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Baker AC, Glynn PW, Riegl B. Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci. 2008;80:435–471. doi: 10.1016/j.ecss.2008.09.003. [DOI] [Google Scholar]
- Baptista M, Lopes VM, Pimentel MS, Bandarra N, Narciso L, Marques A, Rosa R. Temporal fatty acid dynamics of the octocoral Veretillum cynomorium. Comp Biochem Physiol B: Biochem Mol Biol. 2012;161:178–187. doi: 10.1016/j.cbpb.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, Bruckner A, Chiriboga A, Cortes J, Delbeek JC, DeVantier L, Edgar GJ, Edwards AJ, Fenner D, Guzman HM, Hoeksema BW, Hodgson G, Johan O, Licuanan WY, Livingstone SR, Lovell ER, Moore JA, Obura DO, Ochavillo D, Polidoro BA, Precht WF, Quibilan MC, Reboton C, Richards ZT, Rogers AD, Sanciangco J, Sheppard A, Sheppard C, Smith J, Stuart S, Turak E, Veron JEN, Wallace C, Weil E, Wood E. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science. 2008;321:560–563. doi: 10.1126/science.1159196. [DOI] [PubMed] [Google Scholar]
- Collins M, Knutti R, Arblaster J, Dufresne J-L, Fichefet T, Friedlingstein P, Gao X, Gutowski WJ, Johns T, Krinner G, Shongwe M, Tebaldi C, Weaver AJ, Wehner M. Long-term Climate Change: Projections, Commitments and Irreversibility. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- Cornelius PFS, Manuel RL, Ryland JS. Hydroids, sea anemones, jellyfish, and comb jellies. In: Hayward PJ, Ryland JS, editors. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press; 1995. pp. 62–135. [Google Scholar]
- Dickson A, Millero FJ. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Research Part A Oceanographic Research Papers. 1987;34:1733–1743. doi: 10.1016/0198-0149(87)90021-5. [DOI] [Google Scholar]
- Dong Y, Miller LP, Sanders JG, Somero GN. Heat-shock protein 70 (Hsp70) expression in four limpets of the genus Lottia: interspecific variation in constitutive and inducible synthesis correlates with in situ exposure to heat stress. Biol Bull. 2008;215:173–181. doi: 10.2307/25470698. [DOI] [PubMed] [Google Scholar]
- Downs CA, Mueller E, Phillips S, Fauth JE, Woodley CM. A molecular biomarker system for assessing the health of coral (Montastraea faveolata) during heat stress. Mar Biotechnol. 2000;2:533–544. doi: 10.1007/s101260000038. [DOI] [PubMed] [Google Scholar]
- Downs CA, Fauth JE, Halas JC, Dustan P, Bemiss J, Woodley CM. Oxidative stress and seasonal coral bleaching. Free Radic Biol Med. 2002;33:533–543. doi: 10.1016/S0891-5849(02)00907-3. [DOI] [PubMed] [Google Scholar]
- Downs CA, McDougall KE, Woodley CM, Fauth JE, Richmond RH, Kushmaro A, Gibb SW, Loya Y, Ostrander GK, Kramarsy-Winter E. Heat-stress and light-stress induce different cellular pathologies in the symbiotic dinoflagellate during coral bleaching. PLoS One. 2013;8:e77173. doi: 10.1371/journal.pone.0077173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M, Richardson AJ. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–884. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Flores-Ramírez LA, Liñán-Cabello MA. Relationships among thermal stress, bleaching and oxidative damage in the hermatypic coral, Pocillopora capitata. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:194–202. doi: 10.1016/j.cbpc.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Freiwald A, Fosså JH, Grehan A, Koslow T, Roberts JM. Cold-water coral reefs. Cambridge: UNEP-WCMC; 2004. [Google Scholar]
- Gabay Y, Fine M, Barkay Z, Benayahu Y. Octocoral tissue provides protection from declining oceanic pH. PLoS One. 2014;9:e91553. doi: 10.1371/journal.pone.0091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn PW. Coral reef bleaching: facts, hypotheses and implications. Glob Chang Biol. 1996;2:495–509. doi: 10.1111/j.1365-2486.1996.tb00063.x. [DOI] [Google Scholar]
- Gómez CE, Paul VJ, Ritson-Williams R, Muehllehner N, Langdon C, Sánchez JA. Responses of the tropical gorgonian coral Eunicea fusca to ocean acidification conditions. Coral Reefs. 2015;34:451–460. doi: 10.1007/s00338-014-1241-3. [DOI] [Google Scholar]
- Griffin SP, Bhagooli R. Measuring antioxidant potential in corals using the FRAP assay. J Exp Mar Biol Ecol. 2004;302:201–211. doi: 10.1016/j.jembe.2003.10.008. [DOI] [Google Scholar]
- Griffin SP, Bhagooli R, Weil E. Evaluation of thermal acclimation capacity in corals with different thermal histories based on catalase concentrations and antioxidant potentials. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:155–162. doi: 10.1016/j.cbpa.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Grottoli AG, Martins PD, Wolkins MJ, Johnston MD, Warner ME, Cai W-J, Melman TF, Hoadley KD, Pettay DT, Levas S, Schoepf V. Coral physiology and microbiome dynamics under combined warming and ocean acidification. PLoS One. 2018;13:e0191156. doi: 10.1371/journal.pone.0191156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: the first enzymatic in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Harvey BP, Gwynn-Jones D, Moore PJ. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecology and Evolution. 2013;3:1016–1030. doi: 10.1002/ece3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegh-Guldberg O, Cai R, Poloczanska ES, Brewer PG, Sundby S, Hilmi K, Fabry VJ, Jung S. The ocean. In: Barros VR, Field CB, Dokken DJ, Mastrandrea MD, Mach KJ, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL, editors. Climate change 2014: impacts, adaptation, and vulnerability. Part B: regional aspects. Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2014. pp. 1655–1731. [Google Scholar]
- Hofmann GE, O'Donnell MJ, Todgham AE. Using functional genomics to explore the effects of ocean acidification on calcifying marine organisms. Mar Ecol Prog Ser. 2008;373:219–225. doi: 10.3354/meps07775. [DOI] [Google Scholar]
- Inoue S, Kayanne H, Yamamoto S, Kurihara H. Spatial community shift from hard to soft corals in acidified water. Nat Clim Chang. 2013;3:683–687. doi: 10.1038/nclimate1855. [DOI] [Google Scholar]
- Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso G-P. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Chang Biol. 2013;19:1884–1896. doi: 10.1111/gcb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledger PW, Franc S. Calcification of the collagenous axial skeleton of Veretillum cynomorium Pall. (Cnidaria: Pennatulacea) Cell Tissues Res. 1978;192:249–266. doi: 10.1007/BF00220743. [DOI] [PubMed] [Google Scholar]
- Lesser MP. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol. 2006;68:253–278. doi: 10.1146/annurev.physiol.68.040104.110001. [DOI] [PubMed] [Google Scholar]
- Lesser MP. Oxidative stress in tropical marine ecosystems. In: Abele D, Zenteno-Savín T, Vazquez-Medina J, editors. Oxidative stress in aquatic ecosystems. Chichester: John Wiley & Sons, Ltd; 2011. pp. 7–19. [Google Scholar]
- Lopes AR, Trübenbach K, Teixeira T, Lopes VM, Pires V, Baptista M, Repolho T, Calado R, Diniz M, Rosa R. Oxidative stress in deep scattering layers: heat shock response and antioxidant enzymes activities of myctophid fishes thriving in oxygen minimum zones. Deep-Sea Res I Oceanogr Res Pap. 2013;82:10–16. doi: 10.1016/j.dsr.2013.07.014. [DOI] [Google Scholar]
- López-González PJ, Gili JM, Williams GC. New records of Pennatulacea (Anthozoa: Octocorallia) from the African Atlantic coast, with description of a new species and a zoogeographic analysis. Sci Mar. 2001;65:59–74. doi: 10.3989/scimar.2001.65n159. [DOI] [Google Scholar]
- Lüthi D, Le Floch M, Bereiter B, Blunier T, Barnola J-M. High-resolution carbon dioxide concentration record 650,000-800,000 years before present. Nature. 2008;453:379–382. doi: 10.1038/nature06949. [DOI] [PubMed] [Google Scholar]
- Madeira C, Madeira D, Vinagre C, Diniz M. Octocorals in a changing environment: seasonal response of stress biomarkers in natural populations of Veretillum cynomorium. J Sea Res. 2015;103:120–128. doi: 10.1016/j.seares.2015.07.008. [DOI] [Google Scholar]
- Mehrbach C, Culberson CH, Hawley JE, Pytkowicx RM. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure1. Limnol Oceanogr. 1973;18:897–907. doi: 10.4319/lo.1973.18.6.0897. [DOI] [Google Scholar]
- Mydlarz LD, Jacobs RS. An inducible release of reactive oxygen radicals in four species of gorgonian corals. Mar Freshw Behav Physiol. 2006;39:143–152. doi: 10.1080/10236240600708512. [DOI] [Google Scholar]
- Mydlarz LD, McGinty ES, Harvell D. What are the physiological and immunological responses of coral to climate warming and disease? J Exp Biol. 2009;213:934–945. doi: 10.1242/jeb.037580. [DOI] [PubMed] [Google Scholar]
- Njemini R, Demanet C, Mets T. Comparison of two ELISAs for the determination of Hsp70 in serum. J Immunol Methods. 2005;306:176–182. doi: 10.1016/j.jim.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Pallas PS (1766) Elenchus Zoophytorum sistens generum adumbrations generaliores et specierum cognitarum succinctas descriptiones cum selectis auctorum synonymis. Hagae-Comitum, Apud Petrum van Cleef, The Hague
- Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL. Projecting coral reef futures under global warming and ocean acidification. Science. 2011;333:418–422. doi: 10.1126/science.1204794. [DOI] [PubMed] [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Perez FF, Rios AF, Roson G. Sea surface carbon dioxide off the Iberian peninsula (North Eastern Atlantic Ocean) J Mar Syst. 1999;19(1–3):27–46. doi: 10.1016/S0924-7963(98)00022-0. [DOI] [Google Scholar]
- Pörtner H-O, Karl DM, Boyd PW, Cheung WWL, Lluch-Cota SE, Nojiri Y, Schmidt DN, Zavialov PO. Ocean systems. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, Maccracken S, Mastrandrea PR, White LL, editors. Climate change 2014: impacts, adaptation, and vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Chang. Cambridge: Cambridge University Press; 2014. pp. 411–484. [Google Scholar]
- Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
- R Development Core Team (2016) R: A language and environment for statistical computing, [online]. Available from: https://www.R-project.org. Accessed Dec 2017
- Ritson-Williams R, Ross C, Paul VJ. Elevated temperature and allelopathy impact coral recruitment. PLoS One. 2016;11:e0166581. doi: 10.1371/journal.pone.0166581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche RC, Williams GJ, Turner JR. Towards developing a mechanistic understanding of coral reef resilience to thermal stress across multiple scales. Current Climate Change Reports. 2018;4:51–64. doi: 10.1007/s40641-018-0087-0. [DOI] [Google Scholar]
- Ruzicka RR, Colella MA, Porter JW, Morrison JM, Kidney JA, Brinkhuis V, Lunz KS, Macaulay KA, Bartlett LA, Meyers MK, Colee J. Temporal changes in benthic assemblages on Florida Keys reefs 11 years after the 1997/1998 El Niño. Mar Ecol Prog Ser. 2013;489:125–141. doi: 10.3354/meps10427. [DOI] [Google Scholar]
- Sarazin G, Michard G, Prevot FMP. A rapid and accurate spectroscopic method for alkalinity measurements in sea water samples. Water Res. 1999;33:290–294. doi: 10.1016/S0043-1354(98)00168-7. [DOI] [Google Scholar]
- Soriano-Santiago OS, Liñán-Cabello MA, Delgadillo-Nuño MA, Ortega-Ortiz C, Cuevas-Venegas S. Physiological responses to oxidative stress associated with pH variations in host tissue and zooxanthellae of hermatypic coral Pocillopora capitata. Mar Freshw Behav Physiol. 2013;46:275–286. doi: 10.1080/10236244.2013.827877. [DOI] [Google Scholar]
- Teixeira T, Diniz M, Calado R, Rosa R. Coral physiological adaptations to air exposure: heat shock and oxidative stress responses in Veretillum cynomorium. J Exp Mar Biol Ecol. 2013;439:35–41. doi: 10.1016/j.jembe.2012.10.010. [DOI] [Google Scholar]
- Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, Siqueira MF, Grainger A, Hannah L, Hughes L, Huntley B, Jaarsveld ASV, Midgley GF, Miles L, Ortega-Huerta MA, Peterson AT, Phillips OL, Williams SE. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Tomanek L. Variation in the heat shock response and its implication for predicting the effect of global climate change on species' biogeographical distribution ranges and metabolic costs. J Exp Biol. 2010;213:971–979. doi: 10.1242/jeb.038034. [DOI] [PubMed] [Google Scholar]
- Torda G, Donelson JM, Aranda M, Barshis DJ, Bay L, Berumen ML, Bourne DG, Cantin N, Foret S, Matz M, Miller DJ, Moya A, Putnam HM, Ravasi T, Oppen MJHV, Thurber RV, Vidal-Dupiol J, Voolstra CR, Watson S-A, Whitelaw E, Willis BL, Munday PL. Rapid adaptive responses to climate change in corals. Nat Clim Chang. 2017;7:627–636. doi: 10.1038/nclimate3374. [DOI] [Google Scholar]
- Uchiyama M, Mihara M. Determination of malonaldehyde precursor in the tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- Vander Land J (2008) UNESCO IOC register of marine organisms, a common base for biodiversity inventories. Families and bibliography of keyworks. NNM, Leiden and ETI, Amsterdam
- Wiens M, Ammar MSA, Nawar AH, Koziol C, Hassanein HMA, Eisinger M, Müller IM, Müller WEG. Induction of heat-shock (stress) protein gene expression by selected natural and anthropogenic disturbances in the octocoral Dendronephthya klunzingeri. J Exp Mar Biol Ecol. 2000;245:265–276. doi: 10.1016/S0022-0981(99)00167-7. [DOI] [PubMed] [Google Scholar]
- Yakovleva IM, Baird AH, Yamamoto HH, Bhagooli R, Nonaka M, Hidaka M. Algal symbionts increase oxidative damage and death in coral larvae at high temperatures. Mar Ecol Prog Ser. 2009;378:105–112. doi: 10.3354/meps07857. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 18.5 kb)
(DOCX 104 kb)
(DOCX 13.9 kb)
(DOCX 14.2 kb)