Abstract
Fresh osteochondral allograft (OCA) transplantation is a successful single-stage procedure for the treatment of symptomatic cartilage defects of the knee. Although long-term studies reveal reliable improvements in patient-reported outcome scores and graft survival, the limitations of the procedure include graft availability and timely use prior to expiration. To avoid prolonged surgical wait times and progression of lesion size, some surgeons have employed the use of nonorthotopic grafts (e.g., lateral femoral condyle graft for a medial femoral condyle lesion). Additionally, fresh precut OCA cores can be used for smaller symptomatic lesions, thereby precluding surgical delays associated with donor-recipient size matching. We describe our preferred technique for the use of fresh precut OCA cores for the treatment of small osteochondral defects of the knee. The distinct advantages of this technique include single-stage restoration of the articular surface without the donor site morbidity observed with osteochondral autograft transplantation.
Articular cartilage procedures are being performed with increased frequency throughout the United States, as a recent study revealed a 5% annual incidence growth over the past decade.1 As such, there has been a corresponding rise in the number of fresh osteochondral allograft (OCA) transplantation procedures, and graft use has increased by more than 500%.2 A systematic review of OCA transplantation revealed reliable improvements in patient-reported outcomes and a graft survival rate of 78.7% at 10 years. In the young athletic population, OCA transplantation has also achieved a 75.2% rate of return to sport and recreational activity at a mean follow-up of 6 years, thus lending strong support to the use of OCA for the treatment of focal defects.3 OCA transplantation facilitates single-stage restoration of the articular surface using a press-fit osteochondral plug with immediate graft stability to support accelerated rehabilitation; however, there are some limitations of the procedure. The current donor-recipient matching process relies on finding an available hemicondylar allograft that shares nearly identical anterior-posterior and medial-lateral dimensions to the patient's condyle to ensure accurate restoration of surface geometry. This practice can lead to long surgical delays, which may result in progression of lesion size and worsening of clinical symptoms. However, a recent image analysis study suggests that nonorthotopic grafts (e.g., a lateral femoral condyle used for a medial femoral condyle lesion) can successfully restore surface contour to within <1 mm of articular step-off for lesions ≤20 mm.4 Moreover, clinical investigation has revealed that patient-reported outcomes and graft survival after nonorthotopic OCA are similar to those after orthotopic OCA, thus suggesting that condyle-specific matching may not be necessary.5 In this article, we describe our preferred surgical approach to small femoral cartilage defects using fresh precut OCA cores to facilitate timely patient treatment and avoid delays associated with graft matching. The surgical indications and contraindications are summarized in Table 1.
Table 1.
Surgical Indications and Contraindications (Absolute and Relative) for Osteochondral Allograft (OCA) Transplantation Using Fresh Precut Cores
| Indications | Contraindications |
|---|---|
| Symptomatic, grade 3 and 4 chondral or osteochondral lesions ≤2.25 cm2 | Relative (to be corrected prior to or concurrently with OCA transplantation) |
| Focal cartilage defects of the femoral condyle, trochlea, or patella | Lower extremity malalignment (defined as weightbearing axis falling through the area of the cartilage defect) |
| Primary cartilage procedure or salvage procedure for prior failed cartilage repair | Ligament injury and associated joint instability |
| Physiologically young, active patients with debilitating symptoms refractory to nonoperative management | Meniscal deficiency |
| Absolute | |
| Kellgren-Lawrence grade 3 or 4 osteoarthritis with joint space narrowing of the affected compartment | |
| Bipolar cartilage lesions | |
| Inflammatory arthritis (rheumatoid, crystal induced, psoriatic) |
Surgical Technique
Patient Positioning and Bone Marrow Aspirate Concentrate Harvest
The patient is placed in the supine position on the operating room table, and general anesthesia is induced after application of a regional nerve block. We prefer to harvest bone marrow aspirate concentrate from the anterior iliac crest, as it facilitates efficient transition of the procedure from the diagnostic arthroscopy to the open arthrotomy. The anterior superior iliac spine is palpated, and the trocar is percutaneously inserted through the skin approximately 3 to 4 cm proximal to this landmark. The crest is palpated to ensure appropriate orientation of the trocar, and it is manually inserted with a mallet. Approximately 60 cc of bone marrow is aspirated and processed using the Arthrex Angel System Centrifuge (Arthrex, Naples, FL).
Diagnostic Arthroscopy
Using standard anterolateral and anteromedial portals, we are able to accurately assess the lesion characteristics (size, location, grade, morphology) and address concomitant intra-articular pathology. It is important to assess the lesion to ensure that it is not larger than the available fresh OCA core. Fresh precut cores are available in 15 × 12- and 10 × 12-mm plugs from JRF Ortho (Centennial, CO). We routinely have the 15-mm size available when planning on using the fresh cores for the treatment of smaller lesions, as magnetic resonance imaging (MRI) can occasionally underestimate the zone of cartilage injury and it is always possible to downsize the graft with available instrumentation. Once the size is confirmed to be adequate for the available core, we proceed with fresh core transplantation.
Recipient Femoral Site Preparation
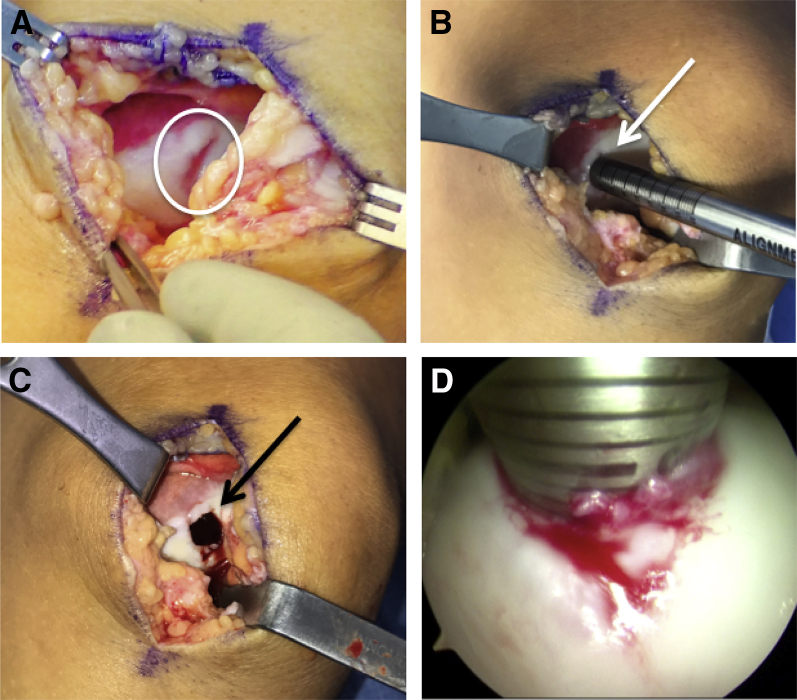
A 6- to 8-cm longitudinal incision is made over the anteromedial or anterolateral knee, incorporating the previously used portal (Video 1). A mini-parapatellar arthrotomy (medial for medial condylar lesions and lateral for lateral condylar lesions) is performed, and the lesion is further assessed for its zone of injury to determine which single-use Arthrex Osteochondral Autograft Transfer System (OATS) instrumentation set should be used. In this case, we found that the lesion measured approximately 10 × 10 mm, so the size 10 set was used (Fig 1A). Alternatively, 6- and 8-mm plugs can be obtained with the Arthrex single-use OATS set, and 12-mm plugs can be obtained using the JRF Ortho OCA instrumentation. The 15-mm plug can be used in its entirety with the Arthrex OCA instrumentation. The alignment rod is oriented perpendicular to the joint surface, and a guide pin is inserted (Fig 1B). A size 10-mm reamer is used to drill the recipient socket to a shallow depth of 6 to 7 mm to facilitate secure press-fit graft fixation but minimize the chance of an immune reaction to bone marrow elements (Fig 1C). Irrigation should be used to prevent heat necrosis. The alignment rod is inserted back into the socket, and by using the arthroscope, we can accurately measure the depth at 12, 3, 6, and 9 o'clock (Fig 1D). The socket is irrigated to remove all bone debris and a No. 15 blade scalpel is used to sharply excise loose cartilage at the periphery to ensure easy graft seating during implantation.
Fig 1.
(A) Intraoperative photograph of a left knee showing a 10 × 10-mm grade 4 chondral lesion of the medial femoral trochlea. (B) The alignment rod is placed over the defect to aid in selection of an appropriately sized single-use Osteochondral Autograft Transfer System (OATS) set. In this particular case, a size 10-mm Arthrex OATS harvester was used, as it covered the entire defect. (C) A guide pin is inserted in a perpendicular fashion and over-reamed to a depth of 6 to 7 mm. Although the amount of reamed bone should be minimized, the depth of drilling should be based on the presence of bone cysts or other associated bony pathology. The final reamed socket is shown. (D) Given the small arthrotomy and potential difficulty with visualizing the precise depth of the socket using the small alignment guide, the arthroscope can be used to measure the final socket at desired locations. We typically obtain depth measurements at 12-, 3-, 6-, and 9-o'clock positions.
Fresh Core Preparation and Implantation
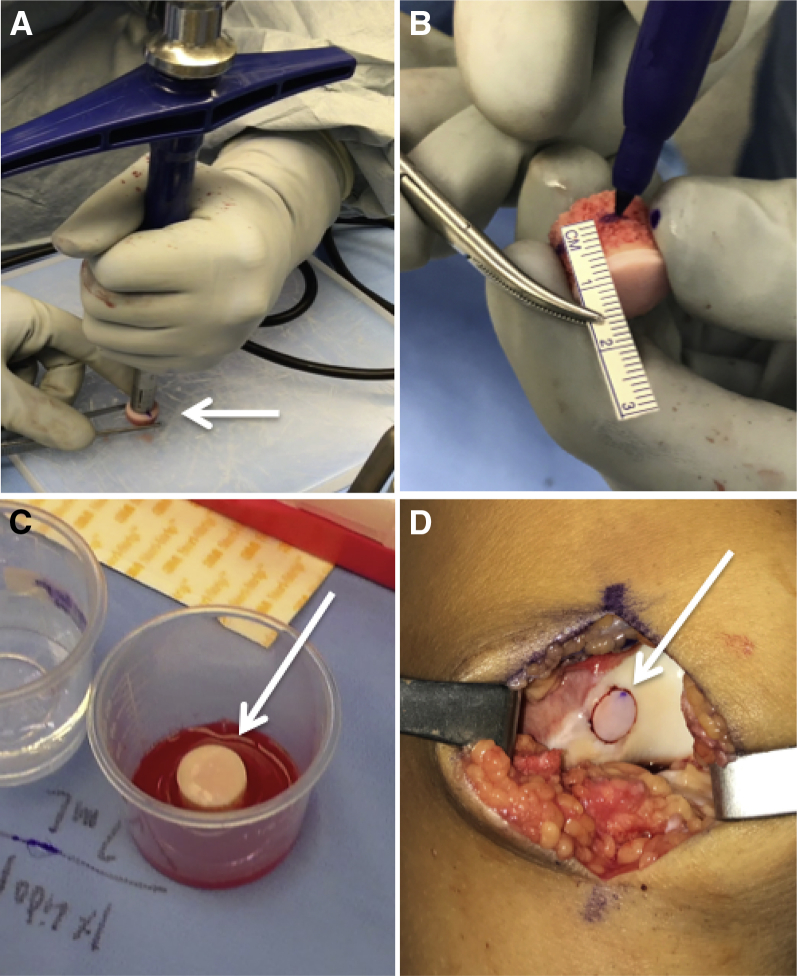
The fresh core is removed from the media and placed on a large preparation board. The 12-o'clock area is marked for orientation, and the size 10 donor harvester is centered on the graft in a perpendicular fashion and impacted until it is flush with the board (Fig 2A). The harvester is gently removed with the graft inside, and the plug is extracted using the core extruder knob. A ruler and skin marker is used to accurately size the length of the graft at the predetermined locations (Fig 2B). The graft is then treated with pulsatile lavage for a minimum of 2 minutes to remove all bone marrow elements. Finally, the graft is left to soak in the bone marrow aspirate concentrate on the back table for a minimum of 10 to 15 minutes (Fig 2C). The recipient socket is lavaged again, and the alignment rod is placed back into the defect to ensure accurate orientation for graft implantation. Using manual pressure, the graft is inserted and then finally seated flush to the surrounding articular surface with the oversized tamp using gentle strikes with a mallet (Fig 2D). The wound is irrigated and closed in a layered fashion.
Fig 2.
(A) The Osteochondral Autograft Transfer System harvester is placed perpendicular to the center of the precut osteochondral allograft (OCA) core on a preparation board and gently impacted through the entire graft. (B) The cancellous bone of the OCA core is marked at the predetermined depths for the 12-, 3-, 6-, and 9-o'clock positions, and the excess bone is removed with a sagittal saw. (C) The graft is left to soak in bone marrow aspirate concentrate for a minimum of 10 to 15 minutes. (D) Intraoperatively, the graft is placed flush to the surrounding articular surface.
Postoperative Rehabilitation
The patient is allowed to use toe-touch weightbearing with crutches immediately postoperatively in a hinged knee brace and advance to full weightbearing by week 2. Additionally, a continuous passive motion machine is used for a minimum of 4 hours per day for the first 4 weeks. We encourage immediate participation in a supervised rehabilitation program to facilitate rapid quadriceps recovery. Early exercises should focus on progressive hip and core resistance exercises to build proximal strength. The return-to-sport phase begins at approximately 16 to 18 weeks postoperatively with a focus on an interval jogging and plyometric program. Most patients are allowed to return to unrestricted athletic activity at 8 to 10 months postoperatively.
Discussion
OCA transplantation has emerged as a popular option for the treatment of cartilage defects, thus resulting in a large demand for fresh hemicondylar allografts. Standard techniques for graft matching rely on pairing donor-recipient size, laterality, and the condyle, which imposes constraints that can lead to surgical delays. Ultimately, prolonged wait times are a concern in the young, active patient population that typically requires this procedure, as even small full-thickness lesions experience increased force at the base and periphery of the defect and can possibly progress in size with continued weightbearing.6, 7 Fresh OCA cores represent an ideal alternative to standard graft matching for small femoral condyle lesions for myriad reasons. Similar to fresh hemicondylar allografts, these cores provide a mature hyaline cartilage/bone construct with 80% absolute viability in the superficial, middle, and deep chondral layers. Next, in the age of rising health care expenditures, OCA cores provide an economical option to hemicondylar allografts, as they are a fraction of the overall cost (approximately one-fifth for 10-mm and two-fifths for 15-mm cores). In addition, they are readily available to surgeons on request and can even be stored in high-volume surgery centers (1°C-10°C) for up to 35 days, thus allowing convenient scheduling of the operative case and point-of-care treatment for incidental lesions found at the time of routine knee arthroscopy.
Despite clear advantages of this technique, there are some important points to consider relative to hemicondylar allografts (Table 2). First, surgical planning relies on MRI to determine if the estimated size of the lesion is suitable for small OCA cores. MRI can occasionally underestimate the total postdebridement defect area by an average of 65%, and it is possible that the total lesion size may be too large for the small cores.8 Additionally, it is possible that more than 1 lesion is found at the time of arthroscopy, thus requiring additional allograft tissue. For these reasons, we recommend consideration of diagnostic knee arthroscopy if the MRI quality is poor or there is any uncertainty regarding the size of the cartilage lesion. Moreover, we recommend use of the largest graft available (15 mm), as it is always possible to downsize the graft using the aforementioned technique. Last, the surgeon can plan to have more than 1 core available at the time of the procedure, and if any grafts are not used, they may be stored for later use without concern of being discarded before expiration at high-volume cartilage centers. An overview of the advantages and disadvantages of this technique is provided in Table 3. Although we routinely use this technique for small chondral lesions of the femoral condyle, the use of fresh precut cores is relatively new, and future studies investigating clinical outcomes and graft survival relative to hemicondylar allografts are ongoing (Table 3).
Table 2.
Technical Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| Verify accurate preoperative measurement of the cartilage lesion to ensure that a full hemicondylar allograft is not necessary. If necessary, consider a diagnostic arthroscopy prior to OCA transplantation or having more than 1 graft available. | Ensure that the OATS harvester is perpendicular to the graft prior to impaction, as deviation may cause an abnormal fit at the articular surface. |
| Have a 15-mm OCA core available at the time of surgery, as it is always possible to downsize the graft to a smaller size. | Excessive manual impaction of the graft into the recipient socket can result in chondrocyte death. |
| Use the arthroscope to accurately measure the depth of the socket at desired locations. | Excessive reaming of the underlying bone necessitates a larger graft length. The amount of subchondral bone should be minimized to reduce an immune reaction. |
| Soak the OCA core in bone marrow aspirate concentrate prior to implantation, as this may aid in graft healing and biologic incorporation. |
OATS, Osteochondral Autograft Transfer System; OCA, osteochondral allograft.
Table 3.
Advantages and Disadvantages of Using Fresh Precut Osteochondral Allograft (OCA) Cores
| Advantages | Disadvantages |
|---|---|
| OCA cores are readily available, which reduces surgical wait times and facilitates convenient surgical scheduling. | Currently available OCA cores can only treat smaller lesions measuring ≤2.25 cm2. |
| Reduced cost relative to hemicondylar allografts. | Stored OCA cores have a limited shelf life and must be used prior to expiration. |
| OCA cores can be stored at high-volume cartilage centers, facilitating point-of-care treatment for incidental lesions. |
Footnotes
The authors report the following potential conflicts of interest or sources of funding: R.J.W. is a consultant for JRF Ortho. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Demonstration of the use of a 15-mm fresh precut osteochondral allograft core for the treatment of a symptomatic, full-thickness 10 × 10-mm chondral defect of the medial femoral trochlea.
References
- 1.McCormick F., Harris J.D., Abrams G.D. Trends in the surgical treatment of articular cartilage lesions in the United States: An analysis of a large private-payer database over a period of 8 years. Arthroscopy. 2014;30:222–226. doi: 10.1016/j.arthro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 2.McCulloch P.C., Gortz S. Osteochondral allograft transplantation: The rationale and basic science. In: Emans P.J., Peterson L., editors. Developing insights in cartilage repair. Springer; London: 2014. pp. 131–148. [Google Scholar]
- 3.Nielsen E.S., McCauley J.C., Pulido P.A., Bugbee W.D. Return to sport and recreational activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2017;45:1608–1614. doi: 10.1177/0363546517694857. [DOI] [PubMed] [Google Scholar]
- 4.Mologne T.S., Cory E., Hansen B.C. Osteochondral allograft transplant to the medial femoral condyle using a medial or lateral femoral condyle allograft: Is there a difference in graft sources? Am J Sports Med. 2014;42:2205–2213. doi: 10.1177/0363546514540446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Jones K.J., Eliasberg C.D., Pais M.D., Rodeo S.A., Williams R.J. Condyle-specific matching does not improve midterm clinical outcomes of osteochondral allograft transplantation in the knee. J Bone Joint Surg Am. 2017;99:1614–1620. doi: 10.2106/JBJS.16.01542. [DOI] [PubMed] [Google Scholar]
- 6.Schinhan M., Gruber M., Vavken P. Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J Orthop Res. 2012;30:214–220. doi: 10.1002/jor.21521. [DOI] [PubMed] [Google Scholar]
- 7.Lacy K.W., Cracchiolo A., Yu S., Goitz H. Medial femoral condyle cartilage defect biomechanics: Effect of obesity, defect size, and cartilage thickness. Am J Sports Med. 2016;44:409–416. doi: 10.1177/0363546515613517. [DOI] [PubMed] [Google Scholar]
- 8.Gomoll A.H., Yoshioka H., Watanabe A., Dunn J.C., Minas T. Preoperative measurement of cartilage defects by MRI underestimates lesion size. Cartilage. 2011;2:389–393. doi: 10.1177/1947603510397534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demonstration of the use of a 15-mm fresh precut osteochondral allograft core for the treatment of a symptomatic, full-thickness 10 × 10-mm chondral defect of the medial femoral trochlea.