Abstract
Background
Permanent pacemaker implantation is the most common complication after Transcatheter aortic valve replacement (TAVR) and is associated with worse outcomes and mortality. However, its impact on quality‐of‐life (QoL) outcomes remains unknown.
Methods
We included 383 consecutive patients undergoing TAVR from January 2012 to 2016 who completed a baseline Kansas City Cardiomyopathy Questionnaire (KCCQ‐12) health survey. The clinical, laboratory, angiographic, QoL, mortality, and occurrence of poor outcomes (KCCQ‐12 score < 45 or KCCQ decrease of ≥10 points) were obtained.
Results
The mean age was 83 ± 8 years, 51% were men, and majority were Caucasians (n = 364, 95%). Permanent pacemaker (PPM) was implanted in 11.5% of patients post‐TAVR. PPM patients were more likely to have prior conduction disease including RBBB (25% vs 12%, P = .02) and PQ interval >250 ms (11% vs 5%, P = .07). One‐month median KCCQ‐12 scores were significantly lower among PPM patients (84.7 vs 68.8, P = .04), but did not differ significantly at 1‐year (86.5 vs 90.6, P = .5) post‐TAVR. Occurrence of poor outcomes did not differ significantly among those with or without PPM at 1 month (11% vs 7%, P = .39) and 1 year (13% vs 9%, P = .45), respectively. However, patients with poor QoL outcomes at 1 month post‐TAVR also had significantly worse mortality during follow‐up in unadjusted (31.3% vs 4.5%, P < .001) and adjusted (HR = 5.30, 95% [CI: 1.85‐15.22, P = .002])analyses, respectively.
Conclusion
Permanent pacemaker implantation is associated with short‐term reduction in QoL without long‐term implications post‐TAVR. Patients with poor QoL post‐TAVR also have significantly higher mortality.
Keywords: pacemaker, quality of life, transcatheter aortic valve replacement
1. INTRODUCTION
Historically, the management of valvular heart disease using a percutaneous route was limited to balloon angioplasty and was associated with a high rate of restenosis.1 The successful use of transcatheter aortic valve replacement (TAVR) has opened up new horizons in the management of structural valve diseases.2 Several randomized clinical trials have demonstrated the safety and efficacy of TAVR in both high operative risk and subsequently in intermediate risk patients.3, 4, 5 These trials have also demonstrated adverse effects associated with the procedure. Disturbances in cardiac conduction have been identified as a common complication after TAVR, often requiring implantation of a permanent pacemaker (PPM).6 The incidence of PPM implantation varies based on the type of valve used and ranges from 5% to 12%.7, 8, 9 PPM implantation after TAVR has been associated with increased hospital length of stay, increased risk of mortality, and heart failure readmissions.10 A cost analysis from the FRANCE registry showed that PPM implantation after TAVR was associated with a 36% increase in procedural costs and increased in‐hospital costs compared to surgical aortic valve replacement.11, 12
A recent study highlighted the importance of measuring quality‐of‐life (QoL) and health status outcomes in patients undergoing TAVR.13 Their study found that functional outcomes are more desired among patients than life expectancy, despite multiple comorbid conditions and advanced age among the cohort. Hence, in addition to measuring survival and occurrence of major adverse cardiac events (MACE), assessment of QoL and health status outcomes is paramount in this population. The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a patient‐reported disease‐specific health status survey that has been validated for assessment of functional status and QoL in patients undergoing TAVR.13, 14 Changes in KCCQ score have also been shown to correspond well to New York Heart Association (NYHA) functional status and extent of clinical improvement.15 However, it remains unclear if patients undergoing PPM after TAVR are likely to have improvement in QoL and overall health status.
To identify this outcome, our study aims to compare QoL outcomes in patients post‐TAVR receiving pacemaker implantation to those without; while also determining factors that predict poor QoL outcomes and correlate that to the survival rates among the cohort at our tertiary care referral center. The study has the following key objectives: (i) to compare QoL outcomes among patients undergoing TAVR who did and did not undergo PPM implantation; (ii) to determine predictors of poor QoL outcomes among patients undergoing TAVR; (iii) to determine if patients experiencing poor QoL also have poor survival post‐TAVR.
2. METHODS
2.1. Study population
The study population was obtained from the prospective TAVR database, which comprised of consecutive patients who presented to our tertiary care center (Gates Vascular Institute, Buffalo, NY) and underwent TAVR from January 2012 to July 2016 and reviewed retrospectively. Those patients who had undergone prior PPM implantation were excluded from the study. All data were collected using the standardized definitions, which conforms to the standards of the Society of Thoracic Surgeons and American College of Cardiology's National Transcatheter Valve Therapy (TVT) registry.16 All patients underwent routine follow‐up post‐TAVR, including KCCQ questionnaire during each visit, at 30 days and 1 year.
2.2. Clinical characteristics
Baseline demographic data were collected on all the patients in the study. Additionally, relevant clinical variables including comorbid conditions and home medications were collected. Detailed procedural characteristics, laboratory data, and prior‐noninvasive testing, including a routine electrocardiogram, were collected. Electrocardiographic (ECG) data obtained prior to TAVR included presence of a left bundle branch block (LBBB), right bundle branch block (RBBB), QRS duration, PQ interval, and the presence of atrial fibrillation. Post‐TAVR PPM implantation and occurrence of postprocedure arrhythmia were recorded as well.
2.3. Society of thoracic surgeons score
The Society of Thoracic Surgeons (STS) score for each patient was also included. The STS risk score is used to assess the risk of morbidity and mortality for common cardiac surgeries. It is one of the most commonly used risk scores for surgical patients.17 The score is especially important in the TAVR population as most patients who undergo TAVR have an elevated STS score, thus classifying them as high surgical risk, making them a poor candidate for surgical aortic valve replacement.18 Thus, the STS scores are one of the major factors considered in assessing patients for TAVR candidacy.
2.4. Quality of life assessment
The QoL assessment was performed using the shortened KCCQ‐12 questionnaire. The KCCQ is an international standard for quantification of disease‐specific health status in patients with heart failure. The 23‐item KCCQ consisted of 7 domains: physical limitations, symptom stability, symptom frequency, symptom burden, self‐efficacy, QoL, and social limitations.19 Subsequently, a short (12‐item) KCCQ was developed retaining the physical limitation, symptom frequency, QoL, and social limitation domains. The KCCQ‐12 was also validated in both stable and acute heart failure recovery patients.20 The KCCQ‐12 summary score ranges from 0 to 100 points, with 100 points being the highest QoL and health status. The KCCQ‐12 variables are routinely collected and reported as part of the TVT registry at the time of presentation and during subsequent follow‐up visits at 30 days and 1 year, respectively.
We defined poor QoL outcome as a KCCQ‐12 score < 45 or KCCQ decrease of ≥10 points post‐TAVR. We used this definition which has been previously validated and corresponds to approximately NYHA Class III symptoms or better and clinically meaningful decline in QoL post‐TAVR (≥10 points).14
2.5. Outcomes assessment
The occurrence of all‐cause mortality post‐TAVR was evaluated in all patients up to 1‐year postprocedure. Mortality data were obtained from the electronic medical records of the patient, U.S. Social Security Death Index, and New York State Death Index records. The occurrence of MACE was defined as a composite outcome of all‐cause mortality, cardiovascular death, stroke, and occurrence of myocardial infarction (MI) within 1‐year postprocedure. The occurrence of MI was defined by symptoms of ischemia and elevations in cardiac biomarkers, as listed by the Joint European Society of Cardiology and the American College of Cardiology.21 Occurrence of stroke was defined as a focal neurologic deficit, from a nontraumatic cause, lasting at least 24 hours and categorized as ischemic (with or without hemorrhagic transformation), hemorrhagic, or of uncertain type (in the case of patients who did not undergo brain imaging or in whom an autopsy was not performed). The State University of New York at Buffalo Institutional Review Board approved the study with a waiver of individual informed consent.
2.6. Statistical analysis
Categorical variables are reported as frequency (%), and comparison between groups was performed by chi‐square tests of independence. Continuous variables are summarized as mean ± SD and compared across groups (PPM and no PPM implantation) using a two‐sample/paired t test or Mann‐Whitney U test as appropriate. Univariate analysis was performed to determine predictors of poor QoL outcomes post‐TAVR. The variables significantly associated with poor QoL in univariate analyses were then entered in the multivariate model, to adjust for confounders associated with PPM implantation and occurrence of poor QoL outcomes post‐TAVR. The cumulative occurrence of all‐cause mortality as a function over time was obtained by the Cox Regression method to obtain the hazards ratio (HR) after ensuring that all the assumptions of the Cox model were met. The event curves were created to compare survival among (i) patients with poor QoL outcomes post‐TAVR compared to (ii) those without poor QoL outcomes post‐TAVR. Statistical analysis was performed using STATA v13.0 (StataCorp, College Station, Texas). A P‐value of <.05 was considered significant.
3. RESULTS
3.1. Study population
We included 383 consecutive patients who underwent TAVR at our institution. The baseline characteristics of the patients are outlined in Table 1. The mean age among all patients was 83 ± 8 years, and equal numbers of patients were men (n = 195, 51%) and women (n = 188, 49%). The majority of patients were Caucasians (n = 364, 95%). Median duration of follow‐up was 9 (Interquartile Range [IQR]: 1, 13) months. The median STS score among all patients was 9.0% (IQR: 6.3%, 11.8%). Clinical characteristics of patients undergoing PPM did not differ significantly among those not requiring PPM post‐TAVR, as shown in Table 1. However, patients undergoing PPM post‐TAVR were more likely to have underlying peripheral arterial disease. Use of AV nodal blockers including beta‐blockers and calcium channel blockers was similar among those with and without PPM post TAVR. Additionally, anti‐platelet agents, vitamin K antagonists (warfarin), and direct oral anticoagulants use were similar among patients with and without PPM post‐TAVR (Table 1).
Table 1.
Baseline clinical characteristics of all patients
| Variables | Pacemaker (n = 44) | No pacemaker (n = 339) | All patients (n = 383) | P‐value |
|---|---|---|---|---|
| Demographics | ||||
| Sex (Male) | 20 (45.5) | 175 (51.6) | 195 (50.9) | .44 |
| Age at procedure | 83 ± 7 | 83 ± 8 | 83 ± 8 | .84 |
| Body mass index | 33.9 (9.2) | 34 (7.7) | 33.9 (7.9) | .96 |
| Race (White) | 40 (90.9) | 324 (95.6) | 364 (95) | .18 |
| Clinical characteristics | ||||
| Smoker | 0 (0) | 10 (2.9) | 10 (2.6) | .25 |
| Diabetes | 15 (34.1) | 124 (36.6) | 139 (36.3) | .75 |
| Hypertension | 43 (97.7) | 316 (93.2) | 359 (93.7) | .34 |
| Systolic heart failure | 8 (19) | 97 (29) | 105 (27.9) | .17 |
| Cerebrovascular disease | 5 (11.4) | 33 (9.7) | 38 (9.9) | .73 |
| Peripheral vascular disease | 25 (56.8) | 131 (38.6) | 156 (40.7) | .02 |
| STS risk score | 8.8 (6.1, 12.5) | 9.0 (6.3, 11.8) | 9.0 (6.3, 11.8) | .96 |
| Prior myocardial infarction | 8 (18.2) | 65 (19.2) | 73 (19.1) | .88 |
| Prior percutaneous coronary intervention | 5 (11.4) | 72 (21.2) | 77 (20.1) | .12 |
| Prior coronary artery bypass surgery | 14 (31.8) | 111 (32.7) | 125 (32.6) | .90 |
| Prior aortic valve replacement | 3 (6.8) | 36 (10.6) | 39 (10.2) | .43 |
| Home medications | ||||
| Aspirin | 32 (72.7) | 208 (61.4) | 240 (62.7) | .14 |
| Clopidogrel | 7 (15.9) | 61 (18) | 68 (17.8) | .73 |
| Direct‐acting oral anticoagulants | 7 (15.9) | 77 (22.7) | 84 (21.9) | .31 |
| Warfarin | 2 (4.5) | 31 (9.1) | 33 (8.6) | .31 |
| Calcium channel blockers | 11 (25) | 71 (20.9) | 82 (21.4) | .54 |
| Beta blockers | 30 (68.2) | 229 (67.6) | 259 (67.6) | .93 |
| Echocardiographic variables | ||||
| Aortic valve area (cm2) | 0.65 (0.5, 0.8) | 0.60 (0.5, 0.7) | 0.60 (0.5, 0.7) | .08 |
| Mean gradient (mm Hg) | 40 (34, 53) | 41 (32, 51) | 41 (33, 51) | .77 |
| Aortic regurgitation (≥moderate) | 5 (11.4) | 64 (18.9) | 69 (18) | .49 |
| Mitral regurgitation (≥moderate) | 9 (20.5) | 79 (22.7%) | 88 (23%) | .75 |
Values are mean ± standard deviation, median (Interquartile range), N (%).
3.2. Procedural characteristics
The transfemoral route was the most common access site (361, 94%) among all patients undergoing TAVR. As shown in Table 2, use of subclavian route was more frequent among patients requiring PPM post‐TAVR. The majority (361, 94.3%) underwent TAVR as an elective procedure. Anesthesia type (general vs moderate sedation) did not differ among those with and without PPM. Use of contrast during procedure, radiation dose (Gray) and duration of radiation exposure also did not differ significantly among those with and without PPM post‐TAVR. Similarly, occurrence of complications including bleeding requiring transfusion was similar among those with and without PPM. Postprocedure creatinine did not differ significantly between the two groups as shown in Table 2. 82% of our patients underwent implantation of the Edwards Sapien Valve including Sapien Valve, Sapien XT, and Sapien 3, the rest of them received the CoreValve. Patients who underwent a CoreValve placement had a higher percentage of post‐TAVR pacemaker implantation compared to those who underwent Edwards Sapien Valve implantation.
Table 2.
Procedural characteristics
| Variables | Pacemaker (n = 44) | No pacemaker (n = 339) | All patients (n = 383) | P‐value |
|---|---|---|---|---|
| Access site | ||||
| Transfemoral | 35 (79.5) | 326 (85.1) | 361 (94) | .004 |
| Anesthesia type | ||||
| Moderate sedation | 24 (54.5) | 153 (45.1) | 177 (46.2) | .22 |
| Procedure indication | ||||
| Elective | 41 (93.2) | 320 (94.4) | 361 (94.3) | .67 |
| Contrast volume (mL) | 180 (140, 235) | 175 (130, 235) | 176 (130, 235) | .70 |
| Fluoroscopy time (min) | 17 (13.4, 25.8) | 16.9 (13.3, 21.9) | 16.9 (13.3, 22) | .51 |
| Fluoroscopy dose (Gray) | 1.1 (0.47, 1.6) | 0.81 (0.52, 1.42) | 0.83 (0.51, 1.43) | .63 |
| Bleeding requiring transfusion | 6 (13.6) | 39 (11.5) | 45 (11.7) | .63 |
| Postprocedure creatinine | 1.12 (0.87, 1.89) | 1.17 (0.9, 1.6) | 1.17 (0.9, 1.6) | .96 |
Values are mean ± standard deviation, median (Interquartile range), N (%).
3.3. Baseline electrocardiographic abnormalities
Table 3 outlines the presence of baseline ECG abnormalities among patients who underwent PPM compared to those not requiring PPM post‐TAVR. Among all patients, atrial fibrillation was the most common abnormality on ECG. LBBB and QRS duration > 150 ms did not differ among those with and without PPM. However, the presence of RBBB on preprocedure ECG was significantly higher among patients who underwent PPM post‐TAVR. Similarly, the presence of a PQ interval > 250 ms was higher in patients undergoing PPM post‐TAVR, as shown in Table 3.
Table 3.
Electrocardiographic characteristics
| Variables | Pacemaker (n = 44) | No pacemaker (n = 339) | All patients (n = 383) | P‐value |
|---|---|---|---|---|
| Left bundle branch block | 3 (6.8) | 36 (11.3) | 39 (10.7) | .60 |
| Right bundle branch block | 11 (25) | 39 (12.3) | 50 (13.8) | .02 |
| QRS duration > 150 ms | 5 (11.4) | 42 (13.1) | 47 (12.9) | .74 |
| PQ interval > 250 ms | 5 (11.4) | 15 (4.7) | 20 (5.5) | .07 |
| Atrial fibrillation | 11 (25) | 152 (44.8) | 163 (42.6) | .01 |
| High grade AV block | 0 (0) | 0 (0) | 0 (0) | NA |
Values are N (%).
3.4. Quality of life outcomes
Among all patients, the median KCCQ‐12 score prior to TAVR was 30.2 (IQR: 18, 43.8) points. Post‐TAVR, there was a significant increase in the median KCCQ‐12 scores at 1 month (84.4 points [IQR: 68.2, 92.7]) and 1 year (86.5 points [IQR: 71.9, 93.8]), respectively. Additionally, median KCCQ‐12 score did not differ significantly (84.1 vs 87.0 points, P = .89) at 1 year post‐TAVR among patients with high‐risk (≥8%) compared to those with intermediate (<8%) risk. Among those patients who underwent PPM, the median KCCQ‐12 score at 1 month post‐TAVR was significantly lower compared to those that did not undergo PPM (68.5 vs 84.7, P = .04). However, at 1 year, the KCCQ‐12 score was similar among those with and without PPM (90.6 vs 86.5, P = .26), Figure 1. Among patients undergoing pacemaker implantation, occurrence of all‐cause mortality (0 vs 3, P = .61), myocardial infarction (0 vs 3, P = .44), and stroke (0 vs 3, P = .44) did not differ at 1 month and 1 year, respectively. Next, poor QoL outcomes defined as a KCCQ‐12 score < 45 or KCCQ decrease of ≥10 points occurred in 33 (8.6%) patients at 1 year post‐TAVR. Occurrence of poor QoL at 1 year post‐TAVR did not differ significantly among patients who did and did not undergo PPM, respectively (13.2% vs 9.3%, P = .45).
Figure 1.
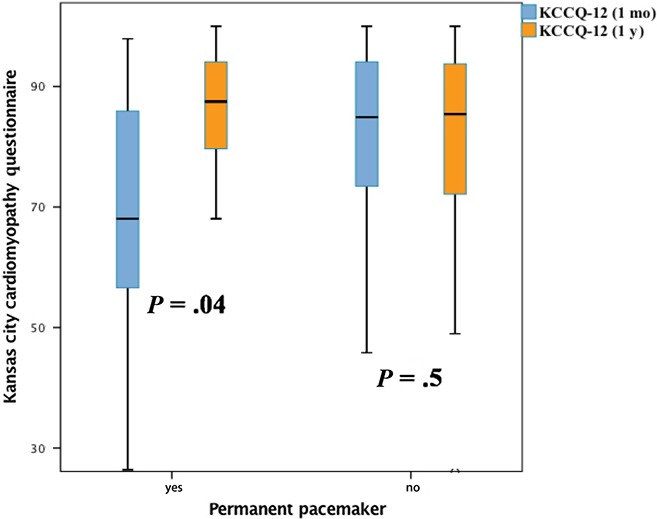
Kansas City Cardiomyopathy Questionnaire (KCCQ)‐12 scores among patient with and without pacemaker at 1 mo and 1 y post‐transcatheter aortic valve replacement (TAVR). The median KCCQ‐12 score at 1 mo post‐TAVR was significantly lower among patients undergoing pacemaker implantation. However, at 1 y, the KCCQ‐12 score was similar among those with and without pacemaker implantation
3.5. Predictors of poor QoL outcomes
Univariate analysis was performed to identify patient characteristics and risk factors that were associated with a poor QoL outcome post‐TAVR and are outlined in Figure 2. Female sex, history of hypertension, prior MI, atrial flutter/fibrillation, high STS risk score, use of general anesthesia, low postprocedure hemoglobin, high BNP, high New York Heart Association functional class, presence of mitral and tricuspid regurgitation, and lower aortic valve area assessed by post‐TAVR echocardiography were significantly associated with poor QoL outcomes. PPM post‐TAVR was not significantly associated with poor QoL outcomes at 1 year both during unadjusted (OR = 1.47, 95% CI: 0.53‐4.07, P = .46) and adjusted analysis (OR = 2.04, 95% CI: 0.68‐6.11, 0.21), respectively.
Figure 2.
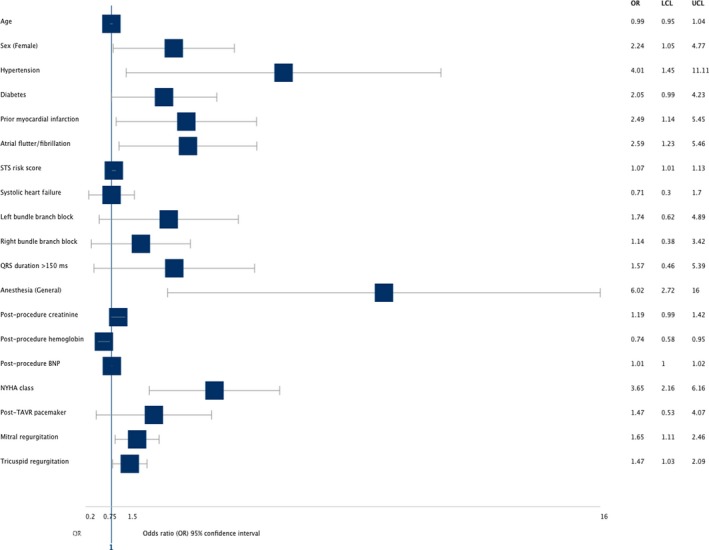
Univariate analysis evaluating the association of variables with poor quality‐of‐life (QoL) outcomes at 1‐y post‐transcatheter aortic valve replacement (TAVR). Female sex, history of hypertension, prior myocardial infarction (MI), atrial flutter/fibrillation, high Society of Thoracic Surgeons (STS) risk score, use of general anesthesia, low postprocedure hemoglobin, high BNP, New York Heart Association (NYHA) class III and higher, presence of mitral and tricuspid regurgitation, and lower aortic valve area post‐TAVR were significantly associated with poor QoL outcomes
3.6. Poor QoL and adverse events
In unadjusted analysis, those patients with poor QoL outcomes at 1 month post‐TAVR follow‐up also had significantly higher all‐cause mortality post‐TAVR (31.3% vs 4.5%, P < .001) and MACE (33.3% vs 5.5%, P < .001). Additionally, patients with poor QoL outcomes at 1 month post‐TAVR had significantly worse survival at 1 year (Hazards Ratio [HR] = 5.87, 95% CI: 2.06‐16.71, P = .001). Cox Regression analysis was used to adjust for risk factors including STS risk score, after ensuring that the test of proportional hazards assumption is satisfied. As shown in Figure 3, after adjusting for STS risk score, patients with poor QoL outcomes one month post‐TAVR continued to have significantly worse survival (HR = 5.30, 95% CI: 1.85‐15.22, P = .002) as well.
Figure 3.
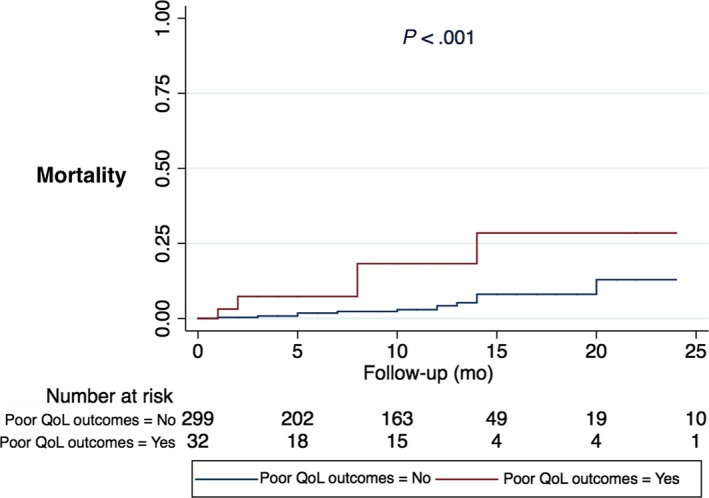
Cox proportional hazards model evaluating poor quality‐of‐life (QoL) outcomes at 1 mo post‐transcatheter aortic valve replacement (TAVR) and all‐cause mortality. After adjusting for STS risk score, patients with poor QoL outcomes 1 mo post‐TAVR continued to have significantly worse survival
4. DISCUSSION
We report a single‐center experience of the impact on QoL outcomes due to PPM post‐TAVR in a prospective cohort. Our study has several key findings: (i) Compared to patients who did not require a PPM, those undergoing implantation post‐TAVR had lower KCCQ‐12 scores in the short‐term, without any significant differences during long‐term (1 year) follow‐up; (ii) After adjusting for relevant predictors of poor QoL, PPM post‐TAVR is not associated with poor QoL outcomes; (iii) patients experiencing poor QoL outcomes post‐TAVR have higher risk of all‐cause mortality and MACE during follow‐up.
4.1. QoL outcomes after TAVR
Definite endpoints like mortality and occurrence of MACE form the cornerstone in the evaluation of any intervention in cardiovascular research. However, as the patient population undergoing TAVR are usually older and have multiple comorbidities, additional outcomes assessing functional and physiological losses limits the maintenance of independence in the elderly population is essential.22 Several patient reporting tools for evaluating QoL have been developed which have been validated in TAVR studies: the Medical Outcomes Trust Short Form 36‐Item Health Survey (SF‐36) and the Short‐Form SF‐12, the Minnesota living with Heart Failure questionnaire (MLHFQ), the EuroQoL 5 (EQ‐5D), and the Kansas City Cardiomyopathy Questionnaire (KCCQ).15, 23, 24, 25, 26 The “KCCQ‐12” questionnaire is a shortened version, which has been validated in both stable and acute heart failure recovery patients20 and is incorporated as a QoL metric that is obtained as part of the TVT registry prior to TAVR and at 1 month, 6 months, and 1 year post‐TAVR, respectively. Although a growing body of evidence has demonstrated improvement in QoL outcomes after TAVR,27, 28 the follow‐up is usually short and patients lost to follow‐up due to poor outcomes may create a potential for bias in these studies. In our study, we demonstrated significant improvement in QoL during both short‐term (1 month) follow‐up and during long‐term follow‐up at 1 year post‐TAVR.
4.2. QoL and pacemaker implantation post‐TAVR
Heart block requiring PPM is the most common complication post‐TAVR6 and is associated with adverse outcomes and increased mortality.8, 10, 11 However, there is no data evaluating the QoL outcomes after PPM in this cohort. Our study is unique in being the first of its kind to assess the impact of postprocedure PPM on QoL outcomes. We demonstrated that patients undergoing PPM had worse QoL during short‐term (1 month) assessment of KCCQ‐12 scores. However, during long‐term (1 year) assessment, we did not find any differences in the QoL among those with and without PPM. We hypothesize that the initial low QoL metrics are secondary to PPM‐related issues (ie, postprocedure pain, limitations in range of motion of the arms, bleeding complications, postprocedure infections, etc.). However, upon subsequent resolution of these issues, patients with PPM actually had slightly higher KCCQ‐12 scores during long‐term follow‐up. We also compared mortality and occurrence of complications including myocardial infarction and stroke at 1 month and 1 year, respectively. Among patients who underwent pacemaker implantation, we found no significant difference in occurrence of mortality and complications during short‐ (1 month) and long (1 year)‐term follow‐up, suggesting that these factors did not play a role in the difference in KCCQ Scores at 1 month and 1 year, respectively. This association remained even after adjusting for other variables that could potentially impact QoL outcomes.
4.3. Pacemaker configuration and poor QoL outcomes post‐TAVR
Short‐term QoL measures may be affected by PPM placement due to extrinsic variables. These variables include but are not limited to (i) depression leading to treatment nonadherence, (ii) increased inflammatory state leading to worsened healing post‐TAVR/post‐PPM, (iii) increased length of hospital stay resulting in nosocomial complications, including systemic infections, and (iv) pacemaker associated complications, such as pacemaker‐site pain, hematoma, and infections. Owing to the transient nature of these extrinsic variables, as represented by the KCCQ score, these differences in measures of QoL eventually resolve at the 1‐year mark in patients with and those without post‐TAVR PPM.
Intrinsic electrocardiac factors, on the other hand, seem to play a major role in long‐term mortality. Due to the anatomical juxtaposition of the aortic valve and the LVOT to the AV conduction system, His Bundle, as well as the left bundle branch, there is an inherent risk of damage to these pathways as a complication of valve deployment. In the presence of underlying pre‐TAVR ECG abnormalities, damage to any of these structures can lead to the development of a high degree AV block, such as second‐degree Mobitz II heart block or complete heart block. As demonstrated in our study, baseline RBBB and increased PQ duration are predisposing variables for requirement of PPM post‐TAVR, with complete heart block being the most common post‐TAVR arrhythmia needing PPM. As seen in other studies, underlying LAFB also predisposes to post‐TAVR PPM.8
Over time, RV pacing can lead to QRS widening and dyssynchrony consistent with LBBB. This dyssynchrony can lead to LV remodeling and ensuing pacemaker‐induced cardiac dysfunction and heart failure, ultimately translating to worsened mortality. Urena et al29 emphasized the detrimental effects of dual‐chamber PPM on LVEF post‐TAVR owing to this cumulative pacing burden. Furthermore, this trial demonstrated that new onset LBBB and low LVEF are predictors of SCD, revealing a negative temporal effect between need for PPM and LVEF.29 Likewise, BLOCK HF trial confirmed the superiority of biventricular pacing over RV pacing in those with AV block.30
4.4. Predictors of poor QoL outcomes post‐TAVR
Several studies and meta analyses have evaluated the predictors of adverse outcomes including PPM post‐TAVR.27, 28 However, the factors contributing to poor QoL post‐TAVR were not evaluated until recently. Cohen et al in a large cohort from the TVT registry demonstrated that older age, higher ejection fraction at baseline, severe lung disease, home oxygen, lower mean aortic valve gradient, prior stroke, diabetes, permanent pacemaker (prior to TAVR), atrial fibrillation, and nonfemoral access were associated with lower 1‐year KCCQ‐OS scores.13 In our cohort, both patient and procedural characteristics including female sex, history of hypertension, prior MI, atrial flutter/fibrillation, high STS risk score, use of general anesthesia, low postprocedure hemoglobin, high BNP, and high New York Heart Association functional class were significantly associated with poor QoL outcomes. As shown in our cohort, a high pre‐TAVR STS score was also shown to be associated with lower postprocedural QoL measures.31
Extent of severity of mitral regurgitation post‐TAVR has been associated with poor QoL as well.32 Taramasso et al33 demonstrated that the presence of residual moderate‐to‐severe paravalvular leak was associated with poor QoL metrics. Likewise in our cohort, the presence of mitral and tricuspid regurgitation and lower aortic valve area assessed by post‐TAVR echocardiography were significantly associated with poor QoL outcomes. One study showed that female sex was associated with worse QoL measures at 3 months; however, it did not remain significant at 12 months post‐TAVR.34 In our cohort, female gender remained significantly associated with poor QoL at 1‐year follow‐up as well. Future studies evaluating gender disparities contributing to these findings are warranted.
4.5. Poor QoL and mortality
Another key finding of our study was the association of poor QoL at 1 month post‐TAVR and increased mortality at 1‐year follow‐up. Even after adjusting for STS risk score, the presence of poor QoL after TAVR remained a strong predictor of increased mortality in our cohort. These findings have several key implications. First occurrence of poor QoL post‐TAVR contributes to further functional decline, thus increasing the risk of MACE and overall mortality. Second, it highlights the importance of QoL as a key predictor of adverse outcomes in patients undergoing TAVR and the need to incorporate QoL in addition to clinically relevant endpoints while evaluating appropriateness for TAVR. Finally, our study highlights the importance of QoL assessment as a key metric irrespective of the estimated risk by traditional risk scores.
4.6. Limitations
Although the data were collected prospectively, the retrospective evaluation of the study hypothesis creates a potential for selection bias. However, all the data are collected using the standardized definitions, which conforms to the standards of the STS ACC'S National TVT registry, which reduces the risk of selection bias. As it is a single‐center experience, the results may not be representative of the general population. The study consists of a relatively small patient cohort especially in the nonagenarian group. However, our study is unique with equal representation of both sexes, which is not commonly seen in cardiovascular research. Although we adjusted for the majority of factors associated with poor QoL outcomes, there may be additional variables that are not routinely measured may be at play and could potentially confound the results. The KCCQ‐12 questionnaire has been validated and extensively utilized for assessment of QoL; however, it may not be comprehensive in estimating all metrics of QoL assessment. Definitions of poor QoL are variable and one single definition may not be applicable to the entire population. Patients lost to follow‐up and excluded may potentially represent a different study population with better/worse clinical characteristics.
5. CONCLUSION
In conclusion, patients undergoing PPM post‐TAVR have short‐term reduction in QoL metrics without any long‐term implications. PPM post‐TAVR is not associated with poor QoL outcomes even after adjusting for other variables contributing to poor QoL. The presence of poor QoL outcomes post‐TAVR is a marker of increased MACE and worse survival at 1 year. Future studies further evaluating the predictors of poor QoL outcomes associated with PPM post‐TAVR are warranted.
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
ACKNOWLEDGEMENTS
We are grateful to all the patients in our registry that made this work possible.
Bhardwaj A, Ramanan T, Sawant AC, et al. Quality of life outcomes in transcatheter aortic valve replacement patients requiring pacemaker implantation. J Arrhythmia. 2018;34:441–449. 10.1002/joa3.12065
Bhardwaj and Ramanan contributed equally to this study.
REFERENCES
- 1. Lababidi Z, Wu JR, Walls JT. Percutaneous balloon aortic valvuloplasty: results in 23 patients. Am J Cardiol. 1984;53:194–7. [DOI] [PubMed] [Google Scholar]
- 2. Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis. First human case description. Circulation. 2002;106:3006–8. [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. [DOI] [PubMed] [Google Scholar]
- 4. Kapadia SR, Leon MB, Makkar RR, et al. 5‐year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–91. [DOI] [PubMed] [Google Scholar]
- 5. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 6. Khatri PJ1, Webb JG, Rodés‐Cabau J, et al. Adverse effects associated with transcatheter aortic valve implantation: a meta‐analysis of contemporary studies. Ann Intern Med. 2013;158:35–46. [DOI] [PubMed] [Google Scholar]
- 7. Laynez A, Ben‐Dor I, Barbash IM, et al. Frequency of conduction disturbances after edwards SAPIEN percutaneous valve implantation. Am J Cardiol. 2012;110:1164–8. [DOI] [PubMed] [Google Scholar]
- 8. Nazif TM, Dizon JM, Hahn RT, et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc Interv. 2015;8:60–9. [DOI] [PubMed] [Google Scholar]
- 9. Maan A, Refaat MM, Heist EK, et al. Incidence and predictors of pacemaker implantation in patients undergoing transcatheter aortic valve replacement. Pacing Clin Electrophysiol. 2015;38:878–86. [DOI] [PubMed] [Google Scholar]
- 10. Fadahunsi OO, Olowoyeye A, Ukaigwe A, et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2016;9:2189–99. [DOI] [PubMed] [Google Scholar]
- 11. Chevreul K, Brunn M, Cadier B, et al. Cost of transcatheter aortic valve implantation and factors associated with higher hospital stay cost in patients of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Arch Cardiovasc Dis. 2013;106:209–19. [DOI] [PubMed] [Google Scholar]
- 12. Osnabrugge RL, Head SJ, Genders TS, et al. Costs of transcatheter versus surgical aortic valve replacement in intermediate‐risk patients. Ann Thorac Surg. 2012;94:1954–60. [DOI] [PubMed] [Google Scholar]
- 13. Arnold SV, Spertus JA, Vemulapalli S, et al. Quality‐of‐life outcomes after transcatheter aortic valve replacement in an unselected population: a report from the STS/ACC transcatheter valve therapy registry. JAMA Cardiol. 2017;2:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arnold SV, Spertus JA, Lei Y, et al. How to define a poor outcome after transcatheter aortic valve replacement: conceptual framework and empirical observations from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Qual Outcomes. 2013;6:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–15. [DOI] [PubMed] [Google Scholar]
- 16. Carroll JD, Edwards FH, Marinac‐Dabic D, et al. The STS‐ACC transcatheter valve therapy national registry: a new partnership and infrastructure for the introduction and surveillance of medical devices and therapies. J Am Coll Cardiol. 2013;62:1026–34. [DOI] [PubMed] [Google Scholar]
- 17. Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high‐risk patientsundergoing aortic valve replacement. J Thorac Cardiovasc Surg. 2008;135:180–7. [DOI] [PubMed] [Google Scholar]
- 18. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597e1607. [DOI] [PubMed] [Google Scholar]
- 19. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 20. Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined–a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21:1502–13. [DOI] [PubMed] [Google Scholar]
- 22. Deutsch MA, Bleiziffer S, Elhmidi Y, et al. Beyond adding years to life: health‐related quality‐of‐life and functional outcomes in patients with severe aortic valve stenosis at high surgical risk undergoing transcatheter aortic valve replacement. Curr Cardiol Rev. 2013;9:281–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Contopoulos‐Ioannidis DG, Karvouni A, Kouri I, Ioannidis JP. Reporting and interpretation of SF‐36 outcomes in randomised trials: systematic review. BMJ. 2009;338:a3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- 25. Guyatt GH. Measurement of health‐related quality of life in heart failure. J Am Coll Cardiol. 1993;22:185A–91A. [DOI] [PubMed] [Google Scholar]
- 26. EuroQol Group . EuroQol–a new facility for the measurement of health‐related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 27. Kim CA, Rasania SP, Afilalo J, Popma JJ, Lipsitz LA, Kim DH. Functional status and quality of life after transcatheter aortic valve replacement: a systematic review. Ann Intern Med. 2014;160:243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lange R, Beckmann A, Neumann T, et al. Quality of life after transcatheter aortic valve replacement: prospective data from GARY (German Aortic Valve Registry). JACC Cardiovasc Interv. 2016;9:2541–54. [DOI] [PubMed] [Google Scholar]
- 29. Urena M, Webb JG, Tamburino C, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation. 2014;129:1233–43. [DOI] [PubMed] [Google Scholar]
- 30. Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–93. [DOI] [PubMed] [Google Scholar]
- 31. Amonn K, Stortecky S, Brinks H, et al. Quality of life in high‐risk patients: comparison of transcatheter aortic valve implantation with surgical aortic valve replacement. Eur J Cardiothorac Surg. 2013;43:34–41; discussion 41–2. [DOI] [PubMed] [Google Scholar]
- 32. Nombela‐Franco L, Ribeiro HB, Urena M, et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J Am Coll Cardiol. 2014;63:2643–58. [DOI] [PubMed] [Google Scholar]
- 33. Taramasso M, Latib A, Cioni M, et al. Quality of life improvement is maintained up to two years after transcatheter aortic valve implantation in high‐risk surgical candidates. EuroIntervention. 2012;8:429–36. [DOI] [PubMed] [Google Scholar]
- 34. Fairbairn TA, Mather AN, Bijsterveld P, et al. Diffusion‐weighted MRI determined cerebral embolic infarction following transcatheter aortic valve implantation: assessment of predictive risk factors and the relationship to subsequent health status. Heart. 2012;98:18–23. [DOI] [PubMed] [Google Scholar]


