Abstract
Background
ROCKET AF and its East Asian subanalysis demonstrated that rivaroxaban was non‐inferior to warfarin for stroke/systemic embolism (SE) prevention in patients with non‐valvular atrial fibrillation (NVAF), with a favorable benefit–risk profile. XANAP investigated the safety and effectiveness of rivaroxaban in routine care in Asia‐Pacific.
Methods
XANAP was a prospective, real‐world, observational study in patients with NVAF newly starting rivaroxaban. Patients were followed at ~3‐month intervals for 1 year, or for ≥30 days after permanent discontinuation. Primary outcomes were major bleeding events, adverse events (AEs), serious AEs and all‐cause mortality; secondary outcomes included stroke/SE. Major outcomes were adjudicated centrally.
Results
XANAP enrolled 2273 patients from 10 countries: mean age was 70.5 years and 58.1% were male. 49.8% of patients received rivaroxaban 20 mg once daily (od), 43.8% 15 mg od and 5.9% 10 mg od. Mean treatment duration was 296 days, and 72.8% of patients had received prior anticoagulation therapy. Co‐morbidities included heart failure (20.1%), hypertension (73.6%), diabetes mellitus (26.6%), prior stroke/non‐central nervous system SE/transient ischemic attack (32.8%) and myocardial infarction (3.8%). Mean CHADS2, CHA2DS2‐VASc and HAS‐BLED scores were 2.3, 3.7 and 2.1, respectively. The rates (events/100 patient‐years [95% confidence interval]) of treatment‐emergent major bleeding, stroke and all‐cause mortality were 1.5 (1.0‐2.1), 1.7 (1.2‐2.5) and 2.0 (1.4‐2.7), respectively. Persistence was 66.2% at the study end.
Conclusions
The real‐world XANAP study demonstrated low rates of stroke and bleeding in rivaroxaban‐treated patients with NVAF from Asia‐Pacific. The results were consistent with the real‐world XANTUS study and ROCKET AF.
Keywords: Asia‐Pacific, bleeding risk, real world, rivaroxaban, stroke prevention
1. INTRODUCTION
The prevalence of non‐valvular atrial fibrillation (NVAF) is growing rapidly in Asia, and it is estimated that the number of patients with NVAF in Asia will be greater than the number in Europe and the USA combined by 2050.1 Data from large clinical trials have clearly indicated differences in the benefit–risk profiles of Asian and Caucasian patients, particularly a higher overall risk of stroke, and greater stroke‐related morbidity and mortality in Asian patients.2, 3, 4 Although treatment with vitamin K antagonists (VKAs) has long been the mainstay of therapy in this setting, warfarin is under‐prescribed in Asian countries; antiplatelet therapy is commonly prescribed instead.4 As an alternative to VKAs, non‐VKA oral anticoagulants (NOACs) could be prescribed in Asian patients with NVAF. The phase III ROCKET AF study showed that rivaroxaban was non‐inferior to warfarin for stroke prevention in patients with NVAF, with a reduced incidence of hemorrhagic stroke and ICH compared with warfarin.5 A subgroup analysis in East Asian patients showed similar efficacy and safety of rivaroxaban vs warfarin,6 as did J‐ROCKET AF, which assessed reduced‐dose rivaroxaban (15 mg once daily [od]) in Japanese patients with NVAF.7 In 2013, the Asia Pacific Heart Rhythm Society (APHRS) added recommendations for the use of NOACs in patients with NVAF.8
Real‐world evidence is increasingly being recognized as an essential component of the clinical evidence base, to complement phase III data in unselected patient populations and provide additional safety information after approval of new drugs. The prospective, observational XANTUS study investigated the safety and effectiveness of rivaroxaban in patients with NVAF in routine clinical practice in Europe, Israel and Canada.9 The results demonstrated low rates of bleeding and stroke/non‐central nervous system (CNS) systemic embolism (SE) in a broad, unselected patient population with NVAF.
Xarelto for Prevention of Stroke in Patients With Atrial Fibrillation in Asia (XANAP) was a sister study to XANTUS and enrolled patients from the Asia‐Pacific region. The main goal of this observational study was to investigate the safety of rivaroxaban use in clinical practice, thereby providing data under real‐life conditions.
2. METHODS
XANAP was a prospective, observational cohort study in unselected patients with NVAF starting rivaroxaban treatment to prevent stroke or non‐CNS SE. The study was part of the XANTUS program and, as such, the study protocol was aligned with that of XANTUS, which is published elsewhere.10
2.1. Study population and data collection
All study sites were in 10 countries across the Asia‐Pacific region: Hong Kong, Indonesia, Malaysia, Pakistan, Philippines, Singapore, South Korea, Taiwan, Thailand and Vietnam. Eligible patients were adults (aged ≥18 years) with a diagnosis of NVAF who provided written informed consent to participate in the study and who started rivaroxaban treatment for the prevention of stroke or non‐CNS SE in accordance with the country‐specific drug approval. Participating investigators were asked to screen all patients with NVAF receiving pharmacological treatment for stroke prevention regardless of the prescribed treatment. This screening documentation occurred before eligible and consenting patients signed the informed consent forms and it was not permissible to collect any patient‐related data from the remaining patients. Each patient was consecutively screened and documented in an anonymous patient log file. To reduce selection bias, enrollment was consecutive and no eligible patients were to be omitted.
2.2. Medication and follow‐up
The decision to treat with rivaroxaban was solely at the discretion of the treating physician. Label‐recommended doses were prescribed in accordance with the country‐specific drug approval. In Taiwan, the approved rivaroxaban dose is 15 or 20 mg od for patients with creatinine clearance (CrCl) >50 mL/min, and 10 or 15 mg od for patients with CrCl 15‐50 mL/min.11 The approved doses of rivaroxaban in the other countries are similar to European label recommendations, ie, 20 mg od for patients with CrCl ≥50 mL/min and 15 mg od for patients with CrCl 15‐49 mL/min.12 Patients were followed up for 1 year or until 30 days after permanent discontinuation of rivaroxaban (if <1 year) and investigators were asked to collect data at approximately 3‐month intervals.
2.3. Study outcomes
The primary outcomes were related to the safety of rivaroxaban and recorded as treatment‐emergent adverse events (AEs), serious AEs (SAEs), all‐cause mortality and major bleeding events (defined using International Society on Thrombosis and Haemostasis criteria). Secondary outcomes were symptomatic thromboembolic events, non‐major bleeding, treatment satisfaction, healthcare resource (number of healthcare professional visits and hospitalizations), treatment persistence and AE/SAE rates in different NVAF risk factor categories. A Central Adjudication Committee adjudicated the endpoints of major bleeding events, stroke, transient ischemic attack (TIA), non‐CNS SE, myocardial infarction (MI), pulmonary embolism, deep vein thrombosis, atrial thrombus, and all‐cause death.
2.4. Study governance
The study complied with the Declaration of Helsinki and was approved by the appropriate Health Authorities, the independent Ethics Committees and independent Review Boards as required. The study was conducted in accordance with Good Pharmacoepidemiological Practice. An independent academic steering committee oversaw the design, execution and conduct of the study; was responsible for manuscript development; had full access to all of the data; and approved all versions of the manuscript.
Data management and statistical analyses were overseen by the sponsor to keep within Good Clinical Practice standards. The lead statistician oversaw programming and validation of the statistical analyses.
2.5. Statistical analysis
Descriptive statistical analyses were performed on the safety population, which included all patients who took at least 1 dose of rivaroxaban. Events were considered treatment‐emergent if they occurred on or after the first dose of rivaroxaban and up to 2 days after the last dose. Statistical analyses were explorative and descriptive in nature. Incidence proportions (number of patients with events/number of treated patients) and incidence rates (events per 100 patient‐years) are presented with corresponding Pearson–Clopper and Poisson rate 95% confidence intervals (CIs), respectively.
3. RESULTS
3.1. Patient population
A total of 2910 patients were screened between January 2013 and October 2015; 2273 were enrolled in the study. The main reasons for exclusion were because of patient decision (n = 235) and availability of drug (n = 111). All enrolled patients were included in the XANAP safety population (Figure 1). The largest patient populations were from South Korea (844 [37.1%]) and Taiwan (614 [27.0%]). The 8 remaining countries each contributed <10% to the study population. The mean treatment duration (±standard deviation) was 296 days (±129), with most patients (71%) treated with rivaroxaban for >270 days. Persistence with treatment was 66.2% at the end of the study.
Figure 1.
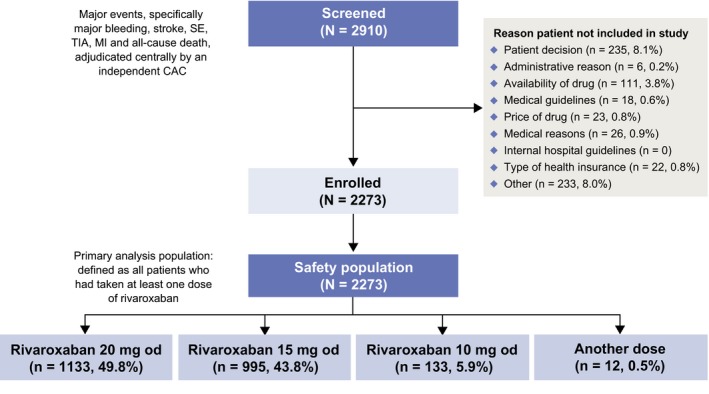
Patient disposition. CAC, Central Adjudication Committee; MI, myocardial infarction; od, once daily; SE, systemic embolism; TIA, transient ischemic attack
The baseline demographics and clinical characteristics of these patients are shown in Table 1. Of note, the proportion of patients who had received prior anticoagulation therapy was high (72.8%), with 35.9% having received prior VKA monotherapy. Mean patient age was 70.5 years; 40.0% of all patients were aged ≥75 years and 58.1% were male. Overall, 49.8% of patients received an initial rivaroxaban dose of 20 mg od; 43.8% and 5.9% received an initial 15 mg od and 10 mg od dose, respectively (Figure 1). Approximately half (48.5%) of patients had missing CrCl data. Of those patients with reported data, 55.0% of patients with a CrCl ≤49 mL/min received rivaroxaban 15 mg od. In patients with a CrCl ≥50 mL/min, rivaroxaban 20 mg od was the most commonly used dose (in 57.4% of patients; Figure S1). Patients had a mean CHADS2 score of 2.3, a mean CHA2DS2‐VASc score of 3.7 and a mean HAS‐BLED score of 2.1. In total, 10.9% of patients had a CHA2DS2‐VASc score of 0 or 1. Co‐morbidities were as follows: 456/2273 (20.1%) patients had congestive heart failure, 1673 (73.6%) had hypertension, 605 (26.6%) diabetes mellitus, 746 (32.8%) prior stroke/non‐CNS SE/TIA and 86 (3.8%) prior MI. There were 504/2273 (22.2%) patients with a first diagnosis of non‐valvular AF, 693 (30.5%) with paroxysmal AF, 576 (25.3%) with persistent AF and 477 (21.0%) with permanent AF.
Table 1.
Baseline demographics and clinical characteristics of patients in XANAP
| Rivaroxaban (N = 2273) | |
|---|---|
| Age, y, mean ± SD | 70.5 ± 10.6 |
| <75 y, n (%) | 1364 (60.0) |
| ≥75 y, n (%) | 909 (40.0) |
| Gender, male, n (%) | 1320 (58.1) |
| Weight, kg, mean ± SD | 66.1 ± 12.8 |
| BMI, kg/m2, mean ± SD | 25.0 ± 4.0 |
| First available CrCl (mL/min), n (%) | |
| <15 | 15 (0.7) |
| ≥15‐<30 | 55 (2.4) |
| ≥30‐<50 | 301 (13.2) |
| ≥50‐≤80 | 543 (23.9) |
| >80 | 257 (11.3) |
| Missing | 1102 (48.5) |
| Existing co‐morbidities, n (%) | |
| Congestive heart failure | 456 (20.1) |
| Hypertension | 1673 (73.6) |
| Diabetes mellitus | 605 (26.6) |
| Prior stroke/non‐CNS SE/TIA, n (%) | 746 (32.8) |
| Prior MI | 86 (3.8) |
| AF type, n (%) | |
| First diagnosed | 504 (22.2) |
| Paroxysmal | 693 (30.5) |
| Persistent | 576 (25.3) |
| Permanent | 477 (21.0) |
| Missing | 23 (1.0) |
| CHADS2 score, mean ± SD | 2.3 ± 1.3 |
| CHA2DS2‐VASc score, mean ± SD | 3.7 ± 1.8 |
| HAS‐BLED score, mean ± SD | 2.1 ± 1.2 |
| Prior antithrombotic therapy, n (%) | |
| Yes | 1449 (63.7) |
| VKA | 813 (35.8) |
| Direct thrombin inhibitor | 149 (6.6) |
| Acetylsalicylic acid | 364 (16.0) |
| Dual antiplatelet therapy | 24 (1.1) |
| Factor Xa inhibitor (excluding rivaroxaban) | 1 (<0.05) |
| Other | 19 (0.8) |
| Multiple | 69 (3.0) |
AF, atrial fibrillation; BMI, body mass index; CNS, central nervous system; CrCl, creatinine clearance; MI, myocardial infarction; SD, standard deviation; SE, systemic embolism; TIA, transient ischemic attack; VKA, vitamin K antagonist.
3.2. Outcomes
Overall, 1.2% (95% CI 0.8‐1.7) of the cohort of 2273 patients had adjudicated treatment‐emergent major bleeding events (primary outcome), and the incidence rate was 1.5 events/100 patient‐years (95% CI 1.0‐2.1). There were 4 fatalities (incidence rate: 0.2 events/100 patient‐years; 95% CI 0.1‐0.6) from adjudicated treatment‐emergent major bleeding events. Incidence rates were 0.7 events/100 patient‐years (95% CI 0.4‐1.2) for ICH and 0.5 events/100 patient‐years (95% CI 0.2‐0.9) for gastrointestinal (GI) bleeding. Any treatment‐emergent AEs and SAEs (primary outcome) occurred in 763/2273 (33.6%) and 272/2273 (12.0%) patients, respectively, with corresponding incidence rates of 50.3 events/100 patient‐years (95% CI 46.8‐54.0) and 15.6 events/100 patient‐years (95% CI 13.8‐17.5), respectively. Causes of adjudicated treatment‐emergent deaths (primary outcome) are presented in Table 2. All‐cause death occurred in 36 patients (1.6%), with cardiovascular death being the most common adjudicated cause of death (21 patients [58.3%], including 3 patients [8.3%] who died from non‐hemorrhagic stroke).
Table 2.
Summary of causes of adjudicated treatment‐emergent deaths
| Adjudicated causes of death,a n (%) | Deaths (N = 36) |
|---|---|
| Bleeding | 4 (11.1) |
| Extracranial hemorrhage | 1 (2.8) |
| Intracranial bleeding | 3 (8.3) |
| Cancer | 0 |
| Cardiovascular | 21 (58.3) |
| Cardiac decompensation, heart failure | 7 (19.4) |
| Arrhythmia | 0 |
| MI | 0 |
| Non‐hemorrhagic stroke | 3 (8.3) |
| Sudden or unwitnessed death | 8 (22.2) |
| Venous thromboembolism | 1 (2.8) |
| Other vascular events | 2 (5.6) |
| SE | 0 |
| Infectious disease | 7 (19.4) |
| Other | 3 (8.3) |
| Unexplained | 6 (16.7) |
MI, myocardial infarction; SE, systemic embolism.
Multiple reasons were recorded for the cause of adjudicated treatment‐emergent death of some patients.
Forty‐seven patients had adjudicated treatment‐emergent symptomatic thromboembolic events (secondary outcome) and the incidence rate was 2.6 events/100 patient‐years (95% CI 1.9‐3.4). The incidence rates (number of events/100 patient‐years [95% CI]) for stroke (any), TIA, non‐CNS SE, stroke/non‐CNS SE and MI were 1.7 (1.2‐2.5), 0.2 (0.1‐0.6), 0.1 (0.0‐0.4), 1.9 (1.3‐2.6) and 0.5 (0.2‐0.9), respectively (Table 3).
Table 3.
Adjudicated treatment‐emergent thromboembolic and bleeding events and all‐cause death
| Incidence proportion, % of patients (95% CI) | Incidence rate, number of patients/100 patient‐years (95% CI) | |
|---|---|---|
| Major bleeding | 1.2 (0.8‐1.7) | 1.5 (1.0‐2.1) |
| Fatal | 0.2 (0.0‐0.4) | 0.2 (0.1‐0.6) |
| Bleeding at a critical site | 0.6 (0.3‐1.0) | 0.8 (0.4‐1.3) |
| Intracranial hemorrhage | 0.6 | 0.7 (0.4‐1.2) |
| GI | 0.4 (0.2‐0.8) | 0.5 (0.2‐0.9) |
| Non‐major bleeding events | 8.8 (7.6‐10.0) | 11.2 (9.7‐12.9) |
| Thromboembolic events (stroke, TIA, non‐CNS SE and MI) | 2.1 (1.5‐2.7) | 2.6 (1.9‐3.4) |
| Stroke/non‐CNS SE | 1.5 (1.0‐2.1) | 1.9 (1.3‐2.6) |
| Stroke | 1.4 (1.0‐2.0) | 1.7 (1.2‐2.5) |
| Primary hemorrhagic | 0.4 | NA |
| Primary ischemic | 0.9 | NA |
| Hemorrhagic transformation | 0.1 | NA |
| No hemorrhagic transformation | 0.8 | NA |
| Missing | 0 | NA |
| Non‐CNS SE | 0.1 (0.0‐0.3) | 0.1 (0.0‐0.4) |
| TIA | 0.2 (0.0‐0.4) | 0.2 (0.1‐0.6) |
| MI | 0.4 (0.2‐0.8) | 0.5 (0.2‐0.9) |
| Composite endpoint (stroke/non‐CNS SE, major bleeding and all‐cause death) | 3.4 (2.7‐4.2) | 4.2 (3.3‐5.2) |
| All‐cause death | 1.6 (1.1‐2.2) | 2.0 (1.4‐2.7) |
CI, confidence interval; CNS, central nervous system; GI, gastrointestinal; MI, myocardial infarction; NA, not available; SE, systemic embolism; TIA, transient ischemic attack.
Rates of major bleeding events and stroke/non‐CNS SE generally rose with increasing CHADS2 and CHA2DS2‐VASc scores (Figure 2A,B). No major bleeding events were recorded for patients with a CHA2DS2‐VASc score of 0 or 1, compared with an incidence rate of 1.6 events/100 patient‐years (95% CI 1.1‐2.4) in patients with a score of ≥2. Similarly, there were no recorded incidents of stroke/non‐CNS SE in patients with a CHA2DS2‐VASc score of 0 or 1, rising to an incidence rate of 0.7 events/100 patient‐years (95% CI 0.1‐2.6) in patients with a score of 2, and 3.3 events/100 patient‐years (95% CI 1.6‐6.1) in patients with scores of 6‐9. The incidence of major bleeding increased with age, occurring at a rate of 1.1 events/100 patient‐years (95% CI 0.6‐1.9) in patients aged <75 years and 2.1 events/100 patient‐years (95% CI 1.2‐3.4) in patients aged ≥75 years. The corresponding rates for stroke/non‐CNS SE were 1.6 events/100 patient‐years (95% CI 1.0‐2.6) and 2.2 events/100 patient‐years (95% CI 1.3‐3.6).
Figure 2.
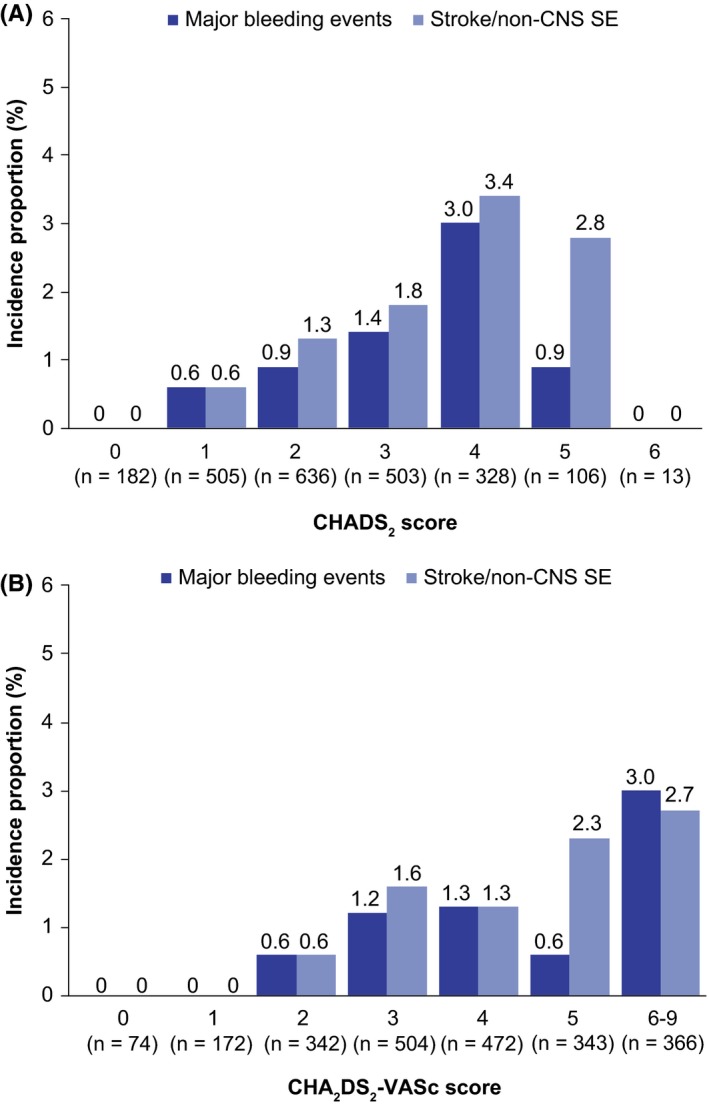
A, Incidence proportion of adjudicated treatment‐emergent stroke/non‐CNS SE and major bleeding by CHADS 2 score; B, Incidence proportion of adjudicated treatment‐emergent stroke/non‐CNS SE and major bleeding by CHA 2 DS 2‐VASc score. CNS, central nervous system; SE, systemic embolism
The cumulative number of adjudicated treatment‐emergent major bleeding events, deaths and symptomatic thromboembolic events increased gradually with time on treatment (Figure 3). Overall, very few patients experienced adjudicated treatment‐emergent major bleeding, death, stroke or non‐CNS SE (4.2 events/100 patient‐years [95% CI 3.3‐5.2]). The incidence rates (number of events/100 patient‐years [95% CI]) of adjudicated treatment‐emergent major bleeding and death were small but numerically higher for patients receiving rivaroxaban 15 mg od compared with those receiving rivaroxaban 20 mg od (1.5 [0.8‐2.6] vs 1.1 [0.5‐2.0] and 2.2 [1.3‐3.5] vs 1.5 [0.8‐2.6], respectively [Figure S2]). Rates of treatment‐emergent outcomes by renal function, and by renal function and dose in patients receiving rivaroxaban 15 mg od or 20 mg od, are shown in Figures S3 and S4, respectively. The endpoints of adjudicated treatment‐emergent thromboembolic, and bleeding events and all‐cause death are detailed in Table 3.
Figure 3.
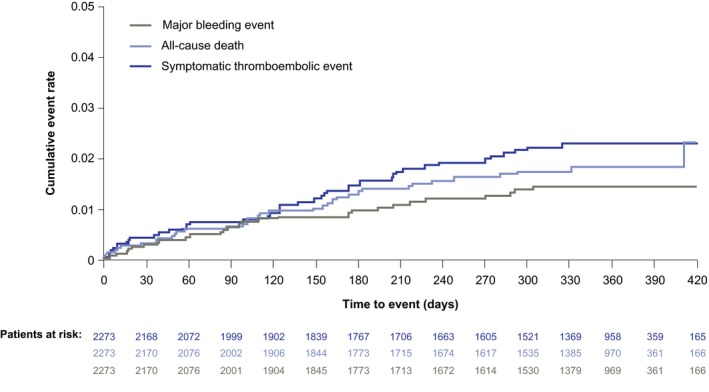
Cumulative rates (Kaplan–Meier) for adjudicated treatment‐emergent all‐cause death, major bleeding events and symptomatic thromboembolic events
3.3. Additional outcomes
In total, 116 patients (5.1%) had at least 1 interruption to rivaroxaban treatment, which was most commonly due to bleeding (31 patients) or surgery (26 patients). The median treatment interruption was 7 days (interquartile range: 3‐23 days). The majority of patients (83.2%) had no unscheduled hospital or general practitioner visits because of the use of rivaroxaban. The majority of patients (60.6%) reported to their physicians that they were either “very satisfied” or “satisfied” with rivaroxaban treatment; a further 26.1% of patients were “neutral”, 6.9% were “unsatisfied”, 0.8% were “very unsatisfied” and data were missing for 5.5%.
4. DISCUSSION
The XANAP study provides insights into the routine clinical use of rivaroxaban in Asian patients with NVAF. XANAP is the first prospective, observational study to describe rivaroxaban use in a broad, real‐world patient population with NVAF in 10 countries of the Asia‐Pacific region. Findings are broadly consistent when cautiously compared with those of the observational sister study (XANTUS) in Europe, Canada and Israel,9 and with the subanalysis of East Asian patients in the phase III ROCKET AF study,6 taking into consideration differences in baseline characteristics. In XANAP, rivaroxaban demonstrated relatively low rates of stroke/non‐CNS SE and low rates of major bleeding, including intracranial and GI bleeding. Over 96% of patients with a mean CHADS2 score of 2.3 receiving rivaroxaban did not experience any of the outcomes of stroke/non‐CNS SE, treatment‐emergent major bleeding or all‐cause death.
The profile of the patient population in XANAP was substantially different from that in XANTUS (Table 4).9 Differences included a higher proportion of patients with prior stroke/non‐CNS SE/TIA, lower mean weight (although weight data were unavailable for 28% of XANAP patients), a higher proportion of patients with moderate or severe renal impairment (with the caveat that CrCl data were not reported for approximately half [48.5%] of patients in XANAP and approximately one‐third [34.4%] of patients in XANTUS) and slightly higher mean CHADS2, CHA2DS2‐VASc and HAS‐BLED scores. These scores were originally derived from Caucasian populations to calculate stroke and bleeding risk in patients with NVAF; however, they have also been shown to be predictive of future stroke and bleeding risk in some Asian populations.4 Based on these scores, NVAF can be calculated to raise the relative risk of stroke fivefold in Caucasian populations, compared with a more modest increase in risk of 2.8‐3.7‐fold, depending on the Asian population studied.4 Finally, persistence at 1 year was lower in XANAP compared with XANTUS (66% vs 80%; Table 4). It is of note that this result is consistent with the treatment persistence reported in a recent large retrospective database analysis of US patients treated with rivaroxaban for stroke prevention (N = 10 878; 60.1% persistence to rivaroxaban therapy at 1 year).13 However, it should be pointed out that the US patient population in this analysis was enrolled in a different healthcare system to those countries in the XANAP studies.13 The lower persistence in XANAP vs XANTUS may reflect lower medication adherence in developing countries, which were highly represented in XANAP.14 Other reasons for the lower persistence rate in XANAP may include issues related to reimbursement, or a lack of follow‐up provision of prescriptions from general practitioners after an initial hospital prescription. Taken together, these differences indicate that the Asian population in XANAP was at higher risk of stroke and bleeding than the European and Canadian population in XANTUS, which is consistent with the observed higher incidence of hemorrhagic stroke and ICH in Asian patients.4 Further comparisons show that XANAP patients were at lower risk of these outcomes than patients in the ROCKET AF East Asian subanalysis6 and J‐ROCKET,7 with CHADS2 scores of 2.3 vs 3.2 and 3.3, respectively (CHA2DS2‐VASc score of 3.7 in XANAP, vs 4.4 in the ROCKET AF East Asian subanalysis; Table 4).
Table 4.
XANAP results in the context of similar clinical trials in different patient populations with atrial fibrillation
| ROCKET AF globala , b 5, 24 (N = 7131)c | J‐ROCKET AFb , d 7 (N = 639)e | ROCKET AF East Asiaa , b 6 (N = 468)f | XANTUSa , g 9 (N = 6784) | XANAPa , g (N = 2273) | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age, y, mean | 73h | 71 | 70 | 71.5 | 70.5 |
| Weight, kg, mean | – | – | 67.3 | 83 | 66 |
| CrCl <50 mL/min, % | 20.7i | 22 | – | 9.4 | 16.3 |
| Congestive heart failure, % | 63 | 41 | 39 | 19 | 20 |
| Hypertension, % | 90 | 80 | 79 | 75 | 74 |
| Diabetes, % | 40 | 39 | 38 | 20 | 27 |
| Prior stroke/SE/TIA, % | 55 | 64 | 65 | 19 | 33 |
| Mean CHADS2 score | 3.5 | 3.3 | 3.2 | 2.0 | 2.3 |
| Mean CHA2DS2‐VASc score | – | – | 4.4 | 3.4 | 3.7 |
| Mean HAS‐BLED score | 2.8j | – | 2.9 | 2.0 | 2.1 |
| Use of rivaroxaban 15 mg od dose, % | 20.7i | – | – | 21 | 44 (+6% 10 mg) |
| Major outcomes (events/100 patient‐years) | |||||
| Major bleeding | 3.6 | 3.0 | 3.4 | 2.1 | 1.5 |
| Fatal | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 |
| In critical organ | 0.8 | 1.5 | 0.7 | 0.7 | 0.8 |
| ICH | 0.5 | 0.8%k | 0.6 | 0.4 | 0.7 |
| GI | 2.0l | 1.3%k | – | 0.9 | 0.5 |
| Stroke/SE | 1.7 | 1.3 | 2.6 | 0.8 | 1.9 |
| Stroke | 1.7 | 1.6%k | 2.6 | 0.7 | 1.7 |
| Ischemic | 1.3 | 1.1%k | 2.1 | 0.5k | 0.9%k |
| Hemorrhagic | 0.3 | 0.5%k | 0.5 | 0.2k | 0.4%k |
| SE | <0.1 | 0.2%k | 0 | 0.1 | 0.1 |
| MI | 0.9 | 0.5%k | 1.0 | 0.4 | 0.5 |
| Thromboembolism (stroke, SE, TIA, MI) | – | – | – | 1.8 | 2.6 |
| All‐cause death | 1.9 | 1.1%k | 2.6 | 1.9 | 2.0 |
| Hemoglobin drop | 2.8 | 1.5 | 3 | 0.9 | 0.1 |
| Transfusions | 1.6 | 0.5 | 1.2 | 0.9 | 0.6 |
| All bleeding | – | – | – | ~17.5 | ~12.7 |
| Major bleeding + clinically relevant non‐major bleeding | 15 | 18 | 21 | – | – |
| Unknown type of stroke | 0.1 | 0 | 0.1m | 0 | 0.2%k |
| AE | 81%k | – | – | 40 | 50.3 |
| SAE | – | 24%k | 23.2%k | 18 | 15.6 |
| 1‐y persistence, % | – | – | – | 80 | 66 |
AE, adverse event; CrCl, creatinine clearance; GI, gastrointestinal; ICH, intracranial hemorrhage; MI, myocardial infarction; od, once daily; SAE, serious adverse event; SE, systemic embolism; TIA, transient ischemic attack.
The rivaroxaban dose was 20 mg od, with the ROCKET AF, ROCKET AF East Asian subanalysis, XANTUS and XANAP studies also having a rivaroxaban 15 mg od dose in patients with renal impairment (30‐49 mL/min in ROCKET AF and East Asian subanalysis; 15‐49 mL/min in XANTUS and XANAP).
The primary outcome for ROCKET AF, ROCKET AF East Asian subanalysis and J‐ROCKET AF was the composite of stroke (ischemic and hemorrhagic) and SE.
Bleeding outcomes in the safety population (n = 7111), efficacy outcomes in the safety, on‐treatment population, which excluded 1 site for violation of Good Clinical Practice (n = 7061).
The rivaroxaban dose was 15 mg od in J‐ROCKET AF, with a rivaroxaban 10 mg od dose in patients with renal impairment (CrCl 30‐49 mL/min).
Bleeding outcomes in the safety, on‐treatment population (n = 639), efficacy outcomes in the per protocol, on treatment population (n = 637).
Bleeding outcomes in the safety, on‐treatment population (n = 466), efficacy outcomes in the intention‐to‐treat population (n = 468).
The primary outcomes for XANTUS and XANAP were related to the safety of rivaroxaban and recorded as treatment‐emergent AEs and SAEs, all‐cause mortality and major bleeding events (defined using International Society on Thrombosis and Haemostasis criteria).
Median age.
Reported in Fox et al.25
Reported in Breithardt et al.26
Incidence proportion.
Reported in Sherwood et al.24
In intention‐to‐treat population.
Overall rates of major bleeding, including major GI bleeding, were lower in patients in XANAP than observed in XANTUS, the ROCKET AF East Asian subanalysis and J‐ROCKET (Table 4).6, 9 The reason for the lower rates of bleeding in XANAP is not completely understood. It is unlikely to simply be a result of the higher proportion of patients in XANAP receiving rivaroxaban 15 mg od, as the findings are consistent with the lower overall bleeding rates observed in rivaroxaban‐treated patients in ROCKET AF from East Asia6 and China,15 compared with patients outside these regions. However, rates of ICH were higher in XANAP compared with XANTUS, but similar to those in the ROCKET AF East Asian subanalysis. This concurs with previously observed higher rates of ICH in Asian populations,16, 17 which are thought to be because of increased cerebral microbleeds in Asian patients4 and can be affected by extremes in body mass index and specific apolipoprotein alleles.16 By contrast, fatal bleeding rates in XANAP were similar to those in XANTUS, the ROCKET AF East Asian subanalysis and J‐ROCKET (Table 4).6, 9
The rates of stroke/non‐CNS SE in XANAP were higher than those in XANTUS, but lower than those observed in the ROCKET AF East Asian subanalysis; this was consistent with the higher stroke risk and proportion of patients with prior stroke/SE/TIA involved in the ROCKET AF East Asian subanalysis (65%, vs 19% in XANAP; Table 4). Stroke is noted in the literature as being a particular risk for Asian patients with NVAF compared with their Caucasian counterparts.4, 18, 19, 20 This is consistent with the higher proportion of patients with prior stroke/non‐CNS SE/TIA at baseline in XANAP (33%, vs 19% in XANTUS). It is possible that this apparently inherent higher risk of stroke in Asian populations contributed to the higher rate of stroke/non‐CNS SE in XANAP. In addition, a potential effect of the ~twofold higher proportion of patients receiving the rivaroxaban 15 mg od dose on increased stroke/SE rates in XANAP cannot be completely excluded. However, it is unclear to what extent the higher proportion of patients receiving the reduced dose would represent inappropriate dosing, as there was a large proportion (48.5%) of patients with unreported CrCl data in XANAP. Additionally, regional differences in the Taiwan label (27% of the XANAP population: recommended dose of rivaroxaban 15 or 20 mg od in patients with CrCl >50 mL/min and rivaroxaban 10 or 15 mg od in patients with CrCl 15‐50 mL/min11) mean that the majority of the Taiwanese patients with CrCl ≥50 mL/min receiving the 15 mg dose were on label. It is also possible that physicians prescribed reduced rivaroxaban 15 mg od to patients with a higher co‐morbidity burden who were more at risk of AEs; in XANTUS, rates of major outcomes were worse in patients receiving the reduced rivaroxaban dose.9 However, it is also of note that the rivaroxaban 15 mg od dose was not associated with higher stroke/SE rates than warfarin in Japanese patients enrolled in the J‐ROCKET AF trial or in patients included in a large retrospective Danish registry analysis.21, 22 Another possible contributor to the higher stroke/SE rate in XANAP was the lower rate of persistence observed in XANAP compared with XANTUS (66% vs 80%), because breaks in anticoagulation could leave patients with NVAF at higher risk of stroke. Of note, the incidence rate of stroke/non‐CNS SE in XANAP of 1.9 events/100 patient‐years is lower than that reported in a recent retrospective cohort study of Taiwanese patients with NVAF; the annual rate in patients taking rivaroxaban (N = 3916) was 3.07 events/100 patient‐years (95% CI 2.12‐4.02) vs 5.62 events/100 patient‐years (95% CI 4.48‐6.76) in those receiving warfarin (N = 5251).23 However, caution must be exercised in comparing data from a retrospective database with the observational study presented here.
Unlike XANTUS, where cancer was one of the main adjudicated causes of death in 19.5% of the patients who died,9 the number of patients with active cancer at baseline, and consequently of patients who died of cancer, was much lower in XANAP than in XANTUS. This may explain why the proportion of cardiovascular deaths was higher in XANAP compared with XANTUS (58.3% vs 41.5%). These results cannot be compared with those of the phase III ROCKET AF study because XANAP, like other observational studies, did not select patients based on strict inclusion criteria. In contrast, phase III studies typically exclude patients with competing co‐morbidities, such as active cancer or short life expectancy. The baseline characteristics of patients enrolled in these trials and the follow‐up periods were different; therefore, it is difficult to make meaningful comparisons of mortality rates between such different types of studies.
4.1. Limitations
XANAP was a real‐world, single‐arm study and, as with any open‐label study, the study design can introduce bias related to knowledge about treatment. Moreover, interference with patient management was not allowed because of the non‐interventional nature of the study. In particular, this resulted in a relatively high proportion of patients (48.5%) with missing renal function data, similar to observations in the XANTUS study (in which CrCl measurements were unavailable for 34.4% of patients).9 Because of the observational nature of the study, it is possible that CrCl was measured but not documented by the prescribing physician for some patients, which would have contributed to the missing data. Additionally, because rivaroxaban prescription was at the discretion of the treating physician, and a relatively important proportion of CrCl data were missing, some patients may not have received the label‐recommended dose of rivaroxaban.
5. CONCLUSIONS
XANAP is the first real‐world, prospective, observational study to describe rivaroxaban use in a broad patient population with NVAF in 10 countries of the Asia‐Pacific region. XANAP had low rates of stroke/non‐CNS SE and major bleeding, including GI and fatal bleeding. When taking differences in baseline risk factors into consideration, findings were consistent with XANTUS and the phase III ROCKET AF study and the subsequent subanalysis in East Asian patients. This is particularly relevant in terms of CHADS2 and CHA2DS2‐VASc scores, which were similar to those in XANTUS, but lower than those in the ROCKET AF East Asian subanalysis.
CONFLICT OF INTEREST
YHK, JS and SM have nothing to disclose. CTT has been a speaker/moderator/story investigator for Bayer, Boehringer Ingelheim, Daiichi Sankyo and MSD. CCW has received consulting fees and honoraria from Bayer, Boehringer Ingelheim, Daiichi Sankyo and Pfizer/Bristol‐Myers Squibb. GV has received consulting fees and honoraria from Bayer, Sanofi and Novartis. MK has advised and spoken for Bayer, Boehringer Ingelheim and Pfizer. OM has been involved in research for Bayer, Boehringer Ingelheim and Pfizer/Bristol‐Myers Squibb, and received consulting fees and honoraria from Bayer, Boehringer Ingelheim and Pfizer/Bristol‐Myers Squibb. LFH has been involved in research for Bayer and Boehringer Ingelheim. THN has been a speaker/moderator/story investigator for AstraZeneca, Bayer, Boehringer Ingelheim, Medtronic and Sanofi. AJC has conducted research, advised and spoken for Bayer, Boehringer Ingelheim, Pfizer/Bristol‐Myers Squibb and Daiichi Sankyo. KS is an employee of Bayer AG; TT was an employee of Bayer AG during the study.
Supporting information
ACKNOWLEDGEMENTS
The XANAP steering committee thanks all patients, caregivers and families who participated in the study as well as the XANAP Investigators and their associated teams. The authors thank Carole Mongin‐Bulewski for editorial assistance in the preparation of the manuscript, with funding from Bayer AG and Janssen Scientific Affairs, LLC.
Kim Y‐H, Shim J, Tsai C‐T, et al. XANAP: A real‐world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation in Asia. J Arrhythmia. 2018;34:418–427. 10.1002/joa3.12073
REFERENCES
- 1. Chiang CE, Wang KL, Lin SJ. Asian strategy for stroke prevention in atrial fibrillation. Europace. 2015;17(Suppl 2):ii31–9. [DOI] [PubMed] [Google Scholar]
- 2. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 3. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 4. Sabir I, Khavandi K, Brownrigg J, Camm AJ. Oral anticoagulants for Asian patients with atrial fibrillation. Nat Rev Cardiol. 2014;11:290–303. [DOI] [PubMed] [Google Scholar]
- 5. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 6. Wong KS, Hu DY, Oomman A, et al. Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke. 2014;45:1739–47. [DOI] [PubMed] [Google Scholar]
- 7. Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J‐ROCKET AF study. Circ J. 2012;76:2104–11. [DOI] [PubMed] [Google Scholar]
- 8. Ogawa S, Aonuma K, Tse HF, et al. The APHRS's 2013 statement on antithrombotic therapy of patients with nonvalvular atrial fibrillation. J Arrhythm. 2014;29:190–200. [Google Scholar]
- 9. Camm AJ, Amarenco P, Haas S, et al. XANTUS: a real‐world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37:1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Camm AJ, Amarenco P, Haas S, et al. XANTUS: rationale and design of a noninterventional study of rivaroxaban for the prevention of stroke in patients with atrial fibrillation. Vasc Health Risk Manag. 2014;10:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bayer Pharma AG . Xarelto® (rivaroxaban) TAIWAN ‐ summary of product characteristics. 2016. Available from http://www.fda.gov.tw/MLMS/ShowFile.aspx?LicId=02025129&Seq=010&Type=9. Accessed March 23, 2017.
- 12. Bayer AG. Xarelto® (rivaroxaban) summary of product characteristics. 2017. Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf. Accessed December 6, 2017.
- 13. Coleman CI, Tangirala M, Evers T. Treatment persistence and discontinuation with rivaroxaban, dabigatran, and warfarin for stroke prevention in patients with non‐valvular atrial fibrillation in the United States. PLoS One. 2016;11:e0157769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poor adherence to long‐term treatment of chronic diseases is a worldwide problem. 2003. Rev Panam Salud Publica. 2003;14:218–21. [PubMed] [Google Scholar]
- 15. Sun Y, Hu D, Stevens S, et al. Efficacy and safety of rivaroxaban versus warfarin in patients from mainland China with nonvalvular atrial fibrillation: a subgroup analysis from the ROCKET AF trial. Thromb Res. 2017;156:184–90. [DOI] [PubMed] [Google Scholar]
- 16. Poon MT, Bell SM, Al Shahi Salman R. Epidemiology of intracerebral haemorrhage. Front Neurol Neurosci. 2015;37:1–12. [DOI] [PubMed] [Google Scholar]
- 17. Tsai CF, Anderson N, Thomas B, Sudlow CL. Comparing risk factor profiles between intracerebral hemorrhage and ischemic stroke in Chinese and white populations: systematic review and meta‐analysis. PLoS One. 2016;11:e0151743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gunarathne A, Patel JV, Gammon B, Gill PS, Hughes EA, Lip GY. Ischemic stroke in South Asians: a review of the epidemiology, pathophysiology, and ethnicity‐related clinical features. Stroke. 2009;40:e415–23. [DOI] [PubMed] [Google Scholar]
- 19. Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011;124:314–23. [DOI] [PubMed] [Google Scholar]
- 20. Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81:264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hori M. The safety and efficacy of rivaroxaban for prevention of stroke in Japanese patients with non‐valvular atrial fibrillation. XXIII Congress of The International Society on Thrombosis and Haemostasis. Kyoto, Japan, 23–28 July 2011, Oral presentation.
- 22. Nielsen PB, Skjøth F, Sogaard M, Kjældgaard JN, Lip GY, Larsen TB. Effectiveness and safety of reduced dose non‐vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2017;356:j510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan YH, Kuo CT, Yeh YH, et al. Thromboembolic, bleeding, and mortality risks of rivaroxaban and dabigatran in Asians with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2016;68:1389–401. [DOI] [PubMed] [Google Scholar]
- 24. Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J Am Coll Cardiol. 2015;66:2271–81. [DOI] [PubMed] [Google Scholar]
- 25. Fox KAA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non‐valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32:2387–94. [DOI] [PubMed] [Google Scholar]
- 26. Breithardt G, Baumgartner H, Berkowitz SD, et al. Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non‐valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur Heart J. 2014;35:3377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


