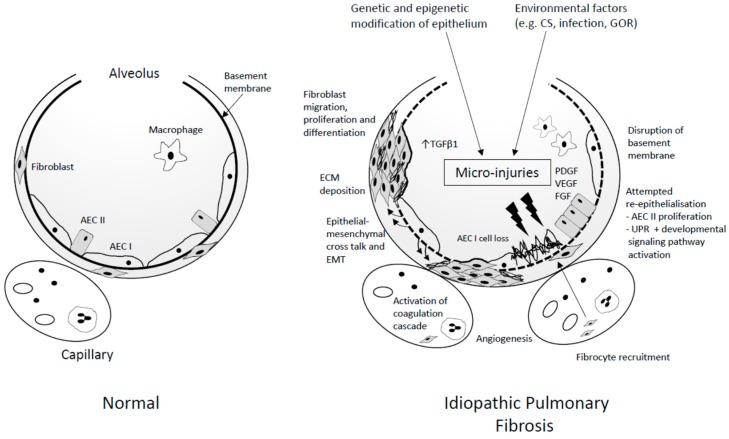
Figure 1.
Schematic view of idiopathic pulmonary fibrosis (IPF) pathogenesis. Genetic and epigenetic phenomenon contribute to the development of an inherently dysfunctional epithelium. This epithelium is susceptible to recurrent micro-injury from environmental exposures (such as cigarette smoke (CS), inhaled dusts, infection, and gastro-oesophageal reflux (GOR)). The inability of the dysfunctional epithelium to regenerate following repetitive injury is a significant juncture in the propagation of IPF. Damage to the epithelium, disrupts the basement membrane and thus the alveolar capillary barrier. Capillary leakage of proteins (including fibrin and fibronectin) into the interstitial and alveolar spaces occurs, with activation of the coagulation cascade and abnormal vascular remodelling as part of the ongoing attempted repair process. Activated epithelial and endothelial cells create a milieu whereby aberrant epithelial–mesenchymal crosstalk, alongside fibrocyte/fibroblast recruitment, migration, proliferation, and differentiation, flourishes. Transforming growth factor β1 (TGFβ1), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF) are some of the pro-fibrotic mediators implicated in these processes. Collections of active fibroblasts and myofibroblasts form fibrotic foci (FF), considered to be at the leading edge of extracellular matrix (ECM) deposition, with progressive lung remodelling and architectural distortion. AEC I/II: alveolar epithelial type I/II cell; UPR: unfolded protein response; EMT: epithelial–mesenchymal transition; UPR: unfolded protein response.