Figure 5.
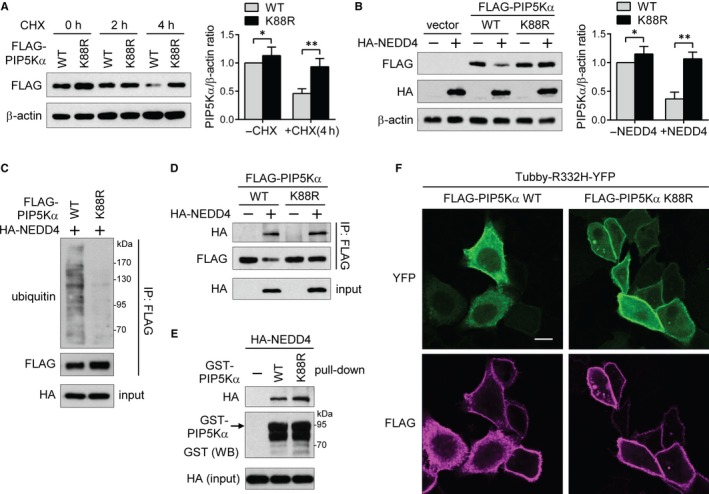
PIP5Kα K88 is the main ubiquitination site for NEDD4. (A) HEK293 cells were treated with or without 10 μmol/L cycloheximide (CHX) for the indicated times at 1 day post‐transfection of WT or K88R FLAG‐PIP5Kα. (B‐D) HEK293 cells were cotransfected with WT or K88R FLAG‐PIP5Kα in the presence or absence of HA‐NEDD4. (A, B) FLAG‐PIP5Kα levels measured by immunoblotting were normalized to β‐actin (a loading control) levels and quantified relative to those in the cells transfected with WT FLAG‐PIP5Kα without CHX (A) or HA‐NEDD4 (B). Values in the graphs are presented as the mean ± SEM. *P < .05, **P < .01. (C, D) FLAG immunoprecipitates and cell lysates (input) were processed for Western blot analysis with the indicated antibodies. (E) Cell lysates expressing HA‐NEDD4 were pulled down with GST‐tagged full‐length WT or K88R PIP5Kα. The bound and input HA‐NEDD4 and GST‐PIP5Kα bait proteins were detected by Western blotting. (F) HeLa cells cotransfected with Tubby‐R332H‐YFP and with either WT or K88R FLAG‐PIP5Kα were immunostained with an anti‐FLAG antibody and followed by staining with the Alexa Fluor 594‐labelled secondary antibody. FLAG immunofluorescence in magenta colour and YFP fluorescence images were captured by confocal microscopy. Scale bar, 10 μm
