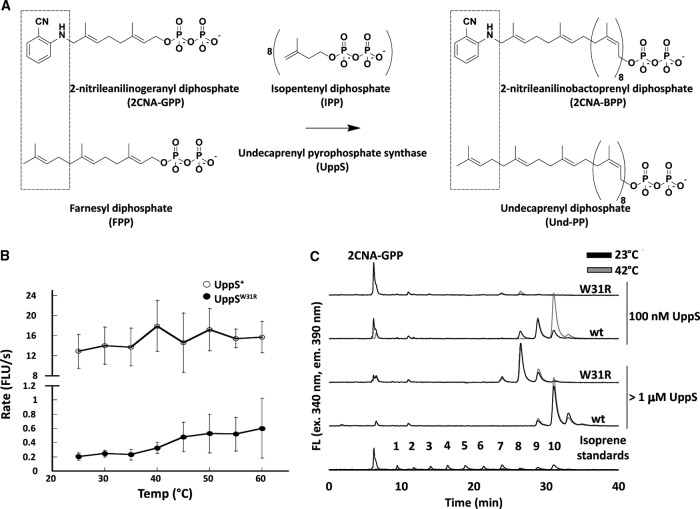
FIG 8.
UppSW31R is less active than UppS at all temperatures. (A) Schematic of fluorescent analogue of FPP. The top schematic is our UppS system in vitro, and the bottom schematic is what occurs naturally in vivo. In the top of the left box is an FPP analog with 2CNA in place of one isoprene unit. The middle shows the reaction of UppS adding 8 additional IPPs to FPP or 2CNA-GPP. The right shows products from the reactions. (B) Reaction mixtures were prepared in a 96-well plate format with 10 μM 2CNA-GPP, 250 μM IPP, and 100 nM UppS cloned from UppS+ or UppSW31R. Fluorescence increase over time was monitored at the given temperatures, and the rate of fluorescence increase was plotted. Points represent the average from two different preparations of protein repeated for a minimum of three times, and error bars represent one standard of deviation from the average. Note that the y axis is broken to allow visualization of the much slower reaction with UppSW31R. (C) UppS+ or UppSW31R in a final volume of 200 μl with 10 μM 2CNA-GPP and 250 μM IPP was incubated at either 23°C or 42°C for 1 h, and the reactions were quenched by adding 50 μl of n-propanol. The reaction products were analyzed by HPLC. UppS+ and UppSW31R were used at either 100 nM or greater than 1 μM. Isoprene standards were prepared by using Bacteroides fragilis UppS in the presence of octyl-thio-glucoside, n-dodecyl-β-d-maltoside, and Tween 20. Standards shown are from three separate separations with each detergent, and peak intensity is normalized for the largest product peak in each mixture. The numbers of isoprene units in each peak were previously confirmed by electrospray ionization-mass spectrometry (ESI-MS). FLU, fluorescent light units.