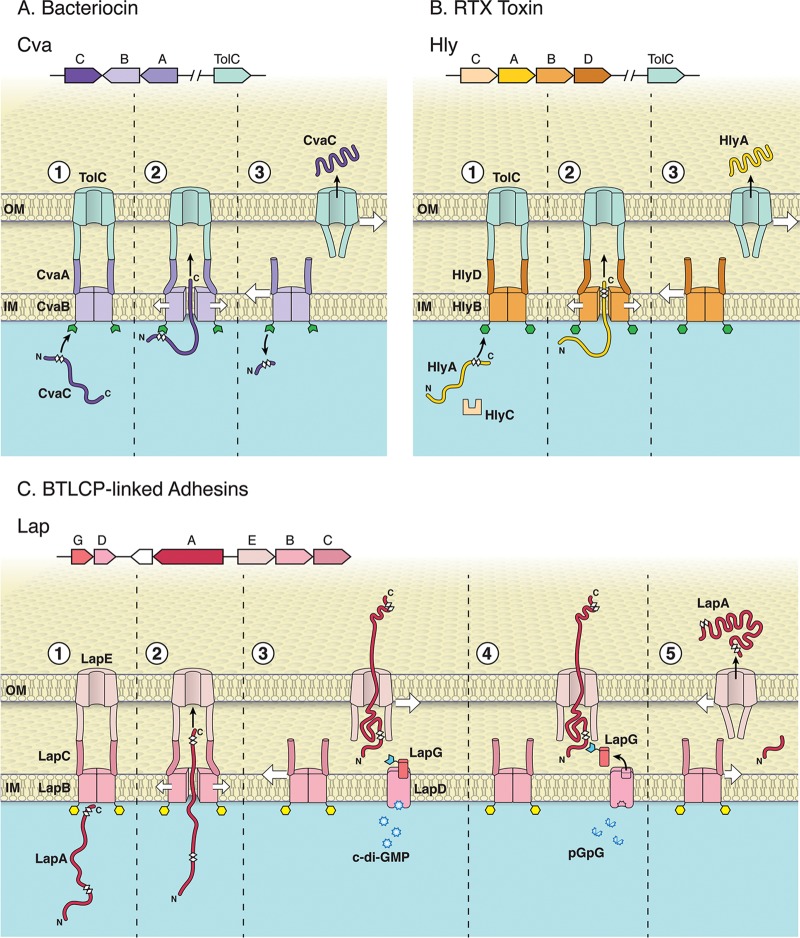
FIG 1.
Overview of three classes of ABC transporters. The T1SS consists of an ABC transporter, MFP, and OM protein. Unfolded T1SS substrates interact with their cognate inner membrane ABC transporter/MFP complex and then recruit and open an OM pore. The inner membrane complex dissociates from TolC following secretion, and TolC returns to the closed state. Variations among these T1SS types are detailed below. The N- and C-terminal portions of the substrates are indicated in the figure, and the white diamonds illustrate the glycine-rich regions of each substrate. (A) Secretion of the bacteriocin CvaC (colisin V); (top) operonic structure of the genes encoding CvaC, CvaB (ABC transporter), and CvaA (MFP) and distally encoded TolC OM pore in E. coli; (bottom) the CvaB protein contains an N-terminal secretion signal with a canonical double glycine motif recognized by CvaB (step 1). CvaB contains a calcium-dependent N-terminal C39 peptidase (green open hexagon) that cleaves CvaC C-terminal to the double glycine site (thereby activating the protein [step 2]) during transport to the extracellular environment. The CvaBA-TolC complex disassembles following secretion of CvaC into the extracellular environment; the closing of the TolC aperture after the secretion complex disassembles is illustrated here and in the subsequent panels (step 3). (B) Secretion of the RTX toxin HlyA; (top) operonic structure of the genes encoding HlyC (accessory acyltransferase that acylates and activates HlyA), HlyA (RTX toxin), HlyB (ABC transporter), HlyD (MFP), and the distally encoded TolC OM pore in E. coli; (bottom) N terminus of HlyB contains a catalytically inactive C39-like domain ([CLD] green hexagon). The CLD of HlyB binds unfolded glycine-rich RTX motifs on the C terminus of HlyA (step 1). The RTX motifs (final ∼50 to 60 aa in HlyA) that engage HlyB, and are ultimately necessary to recruit TolC for secretion, are N-terminal to the secretion signal (step 2). The HlyBD-TolC complex disassembles following secretion of HlyA into the extracellular environment (step 3). (C) Secretion of the BTLCP-linked RTX adhesin LapA in P. fluorescens Pf0-1; (top) operonic structure of the genes encoding LapG (periplasmic calcium-dependent BTLCP that cleaves LapA), LapD (c-di-GMP-sensing effector that sequesters LapG when c-di-GMP is bound), LapA (BTLCP-linked RTX adhesin), LapE (TolC-like OM pore), LapB (ABC transporter), and LapC (MFP); (bottom) LapA secretion requires LapB, which has a CLD distinct from HylB (yellow hexagon [step 1]). LapA is secreted in a C-terminal to N-terminal direction (step 2), but secretion of LapA is stalled during translocation, leaving LapA threaded through LapE with its cleavable N-terminal retention domain localized in the periplasm and multiple adhesive repeats exposed at the cell surface (step 3). LapA is fixed at the cell surface as a biofilm-promoting secretion intermediate when c-di-GMP levels are high and LapD has bound this dinucleotide. c-di-GMP-bound LapD sequesters LapG (open blue hexagon [step 3]), protecting LapA from LapG proteolysis. When c-di-GMP levels are reduced via conversion of this signal to the linear molecule pGpG, LapG is released from LapD (note empty LapG binding site), LapG can cleave LapA at a canonical dialanine site within the retention module (step 4). The cleaved LapA is released from the cell surface, and the unoccupied LapE is now free to interact with substrate-engaged LapBC (step 5). Because the CLD of LapB is distinct from the CLD of HlyB and the C39 domain of CvaB, its role in LapA secretion is unclear. Given the relatedness of these transport systems, the CLD of LapB may bind the glycine-rich regions found at the N or C terminus of LapA (see step 1). (Copyright William Scavone, Kestrel Studio; reproduced with permission.)