Figure 3.
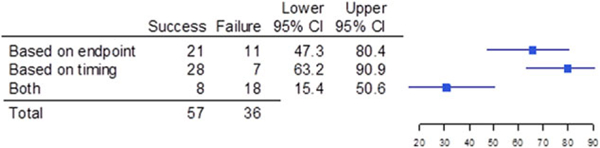
Success and failure of pediatric trials that used a different endpoint than the adult endpoint, broken down to differences in the endpoint or differences in the timing of the evaluation. Shown are the numberofpediatric trials thatwere different based on endpoint,different based on timing of the evaluation, and different based on both endpoint and timing (both); the upper and lower bounds of the 95% confidence intervals (Cls);and a graphical depiction of the confidence intervals.
