Abstract
In light of the current worldwide addiction epidemic, the need for successful therapies is more urgent than ever. Although we made substantial progress in our basic understanding of addiction, reliable therapies are lacking. Since 40-60% of patients treated for substance use disorder return to active substance use within a year following treatment discharge, alleviating the vulnerability to relapse is regarded as the most promising avenue for addiction therapy. Preclinical addiction research often focuses on maladaptive synaptic plasticity within the reward pathway. However, drug induced neuroadaptations do not only lead to a strengthening of distinct drug associated cues and operant behaviors, but also seem to increase plasticity thresholds for environmental stimuli that are not associated with the drug. This form of higher order plasticity, or synaptic metaplasticity, is not expressed as a change in the efficacy of synaptic transmission but as a change in the direction or degree of plasticity induced by a distinct stimulation pattern. Experimental addiction research has demonstrated metaplasticity after exposure to multiple classes of addictive drugs. In this review we will focus on the concept of synaptic metaplasticity in the context of preclinical addiction research. We will take a closer look at the tetrapartite glutamatergic synapse and outline forms of metaplasticity that have been described at the addicted synapse. Finally we will discuss the different potential avenues for pharmacotherapies that target glutamatergic synaptic plasticity and metaplasticity. Here we will argue that aberrant metaplasticity renders the reward seeking circuitry more rigid and hence less able to adapt to changing environmental contingencies. An understanding of the molecular mechanisms that underlie this metaplasticity is crucial for the development of new strategies for addiction therapy. The correction of drug-induced metaplasticity could be used to support behavioral and pharmacotherapies for the treatment of addiction.
Keywords: Addiction, Nucleus Accumbens, Glutamate, tetrapartite synapse, synaptic plasticity, metaplasticity
1. Introduction
The recent addiction epidemic is a social burden that imposes excruciating suffering for families, erodes communities and entails immense socio-economic costs. With more than 90 people dying from opioid overdose every day, drug-related death is currently the leading cause of death among Americans under 50 (U.S. Department of Health and Human Services, 2016). In light of this epidemic the need for successful addiction therapies is more urgent than ever. After 80 years of addiction research we made enormous advances in our knowledge about the reward system and neuroadaptations underlying compulsive drug seeking. We have learned that drug addiction is a chronic neuropsychiatric disorder associated with distinct neurobiological markers rather than a deficit in discipline or will power (Kalivas & Volkow, 2005; Koob & Volkow, 2010). But despite the progress in basic understanding of the disease, reliable pharmacotherapies are still missing.
The first step in an individuals fight against drug addiction is the desire to stop drug use, and there are numerous ways to support patients to take this first step, for example by treating acute withdrawal symptoms (National Institute on Drug Abuse, 2012). However, after successfully achieving abstinence, the biggest challenge for patients is to withstand the persistent vulnerability to relapse, even after many years of abstinence. Within a year following treatment discharge 40-60% of patients treated for substance use disorder return to active substance use (Mclellan, Lewis, O'Brien, & Kleber, 2000). Alleviating this vulnerability to relapse might therefore be the most promising avenue for addiction therapy. Accordingly, a major research goal is to gain deeper understanding of the neurobiology of relapse. The propensity to relapse, despite negative consequences suggests drug-induced deficits in brain regions regulating motivated behaviors to increase positive and decrease negative behavioral outcomes. Recent findings indicate that compulsive drug seeking is controlled by cortico-limbic-striatal circuitry. One central component of this circuit is the nucleus accumbens (NAc). The NAc is conceptualized as the « gatekeeper » of the basal ganglia; it receives glutamatergic input from cortico-limbic areas, as well as dopaminergic input from the midbrain, and is thus ideally positioned to integrate changes in environmental contingencies that shape adaptive behavioral responses (Floresco, 2015). The acquisition of drug-reward associations depends on the convergence of dopamine and glutamate release within the NAc, whereas drug cue induced reinstatement is mostly driven by glutamate (Kalivas & Volkow, 2005; Koob & Volkow, 2010; Scofield, Heinsbroek, et al., 2016).
Experimental research has long focused on how drugs of abuse “hijack” synaptic plasticity in corticostriatal circuitry to change the strength of glutamatergic synaptic transmission in the NAc. However, this aberrant synaptic plasticity not only strengthens distinct drug associated cues and operant behaviors, but also seems to increase plasticity thresholds for environmental stimuli that are not associated with the drug. This form of higher order plasticity, that is not expressed simply as a change in the efficacy of synaptic transmission but as a change in the threshold or rules to induce plasticity, is termed metaplasticity (Abraham & Bear, 1996). Metaplasticity is a common denominator of drug induced neuroadaptations in the brain (Lee & Dong, 2011; Mameli & Luescher, 2011), and can be seen as the gatekeeper for synaptic plasticity. Thus, by setting plasticity rules metaplasticity precludes runaway weakening or strengthening of synaptic transmission and allows the synapses to maintain a functional dynamic range, increasing information storage capacity and ensuring network stability (Fusi & Abbot, 2007).
The weakening of behavioral flexibility to reduce drug seeking behaviors in the presence of negative outcomes is seen in addicts and can be modeled in rodents, and has contributed to a characterization that addiction is a learning disorder (Keramati, Durand, Girardeau, Gutkin, & Ahmed, 2017). Here we will argue that aberrant metaplasticity renders the reward seeking circuitry more rigid and hence less able to adapt to changing environmental contingencies, and that this is a contributing cause of the learning disorder characterized by behavioral inflexibility in drug seeking. Drug induced metaplasticity can be deployed by many different cell signaling and morphological adaptations at glutamatergic synapses. We postulate that targeting these neuroadaptations can restore plasticity thresholds, and thereby normalize computational capacity in reward seeking circuitry. Accordingly, we begin this review by introducing the basic animal models used to study addiction (Section 1.1.) After outlining the connectivity of the NAc within brain reward circuitry (Section 1.2), we provide an overview of synaptic plasticity and metaplasticity (Section 1.3). Then we will take a closer look at glutamatergic synapses, introduce the tetrapartite synapse and outline which forms of metaplasticity can occur and have been described at the addicted glutamatergic synapse (Section 2). Finally we will discuss the different potential avenues for pharmacotherapies that target glutamatergic synaptic plasticity and metaplasticity (Section3).
1.1. Rodent models of reward learning and addiction
Studies on the neurobiological underpinnings of addiction behavior usually employ rodent models because it is necessary to use a species that is capable of performing complex behavioral tasks (self-administration and reinstatement) and has a brain reward system that is sufficiently similar to the human brain. Addiction models can be grouped into the subclass of non-contingent models, where the experimenter delivers the drug and contingent models, where the drug is self-administered by the subject. Non-contingent models are popular, because they are inexpensive and some of the biological changes elicited by non-contingent drug administration are thought to drive addiction. However, self-administration models are believed to simulate the human behavior more accurately, i.e. have greater face validity.
1.1.1. Non-contingent models
Locomotor sensitization
Drug sensitization is defined as the locomotor response induced by acute drug administration, which is progressively augmented with repeated administration and usually persists for weeks or months after the last drug injection. It has been hypothesized that the neuroadaptations that are caused by non-contingent drug exposure and drive locomotor sensitization also contribute to the pathological reward-seeking characteristic of addiction (Robinson & Berridge, 2000).
Conditioned place preference (CPP)
CPP involves a two- or three-chambered apparatus in which the chambers are distinguished by wall color and/or floor texture. The drug is administered over several days in one of these chambers and the ability of addictive drugs to establish learned contextual associations is studied (Tzschentke, 2007).
1.1.2. Contingent models – Self-administration
The acquisition of drug self-administration occurs over the course of several sessions in which an animal learns to conduct an operant response (lever pressing or nose poking), which is reinforced by IV or oral availability of a drug of abuse. A conditioned stimulus (typically a visual (light) and/or auditory (tone) stimulus) occurs simultaneously with the drug administration. In models using limited access, the self-administration sessions are shorter (e.g. 2 hours/10 days) compared to the extended access model (e.g. 6 hours/45 days). Extended access usually results in an increase of drug intake over sessions and is thought to model the escalation of drug use during the development of addiction. The period of self-administration acquisition and maintenance is then followed by a period of withdrawal, where the access to the drug is discontinued. The withdrawal period can be with or without extinction training. During extinction training the animal continues with daily exposure to the operant box but the operant response does not result in cue presentation or drug infusion. During reinstatement, the extinguished operant responding for the drug is reinstated by non-contingent re-exposure to the drug (drug priming), drug-associated cues or stress, however, the operant response remains unrewarded. Reinstatement aims to model stimulus-induced craving prior to achieving drug use. If the animal has not undergone extinction training, return to the drug-paired context will initiate robust drug seeking that may also include cue presentation.
Contingent natural reinforcers
it is important to evaluate and discriminate between neuroadaptations induced by natural reinforcers (i.e. sucrose) and neuroadaptations induced by exposure to drugs of abuse. To this aim, a control group of animals is often trained in the same operant paradigm as for drug delivery in order to control for neuroadaptations produced by operant responding and Pavlovian conditioning.
1.2 Nucleus accumbens connectivity and function
The NAc has been conceptualized as the « gatekeeper » of the basal ganglia and plays an essential role in motivation and reward (Scofield, Heinsbroek, et al., 2016). It receives glutamatergic input from cortico-limbic areas, as well as dopaminergic input from the midbrain, and is therefore ideally positioned to integrate changes in environmental contingencies to shape adaptive behavioral responses (Floresco, 2015). The NAc can be subdivided into a central core (NAcore) and a surrounding shell (NAshell) region, and these sub-regions are involved in distinct reward related tasks. The NAcore receives afferents from the prefrontal cortex (prelimbic, anterior cingulate, anterior insular) the perirhinal and entorhinal cortex, the basolateral amygdala (BLA) and the paraventricular thalamus, and is involved in the learning of reward associated goal-directed behavior (Floresco, 2015) and cue induced drug seeking (Kalivas, 2009). The prelimbic-NAcore projections are necessary to elicit drug primed-, stress- or cue-induced reinstatement of seeking for many classes of drugs of abuse (McFarland & Kalivas, 2001; StefanikS Kupchik, & Kalivas, 2015). The NAshell receives inputs from the infralimbic cortex (Sesack 1989), the BLA and the ventral hippocampus (Britt et al., 2012), and processes the incentive motivational value of reward. Inhibition of the ventromedial NAshell elicits cocaine seeking in rats extinguished from cocaine self administration (Peters, LaLumiere, & Kalivas, 2008), indicating that NAshell activity is necessary to inhibit non-reinforcing or goal-irrelevant behaviors (Floresco, 2015).
Like the dorsal striatum, the NAc network is composed of cholinergic interneurons, fast spiking interneurons, low threshold spiking interneurons and medium spiny neurons (MSNs) (Kreitzer, 2009). While all cell types have specific roles in regulating NAc circuit activity and are involved in motivational tasks (Cachope et al., 2012; Smith et al., 2017; Yu et al., 2017) most studies have focused on MSNs, which comprise 90-95% of all neurons in the striatum and are the sole output neurons from the NAc (R. J. Smith, Lobo, Spencer, & Kalivas, 2013). The GABAergic MSNs can be divided into two subpopulations based on their expression profile for receptors and neuropeptide co-transmitters: D1 receptor–expressing MSNs also co-express dynorphin, substance P, and M4 cholinergic receptors, whereas D2 MSNs express enkephalin, neurotensin, and A2a adenosine (R. J. Smith et al., 2013). Many studies using cell type specific expression of activity regulators such as channel opsins and DREADDs, reveal that D1- and D2-MSNs differently regulate reward behaviors. The consensus of these studies is that D1 MSNs promote drug-seeking behavior and are the major target of drug-induced neuroadaptations, whereas D2 MSNs negatively modulate reward seeking behavior (Calipari et al., 2016; Heinsbroek et al., 2017; Lobo et al., 2010).
Medium spiny neurons in the NAc project either directly or indirectly (via the ventral pallidum) to the ventral mesencephalon. While the dorsal striatum has a relatively strict segregation with D1-MSN contributing to the direct and D2-MSNs to the indirect projections, this is not true for the NAc. For both, the NAcore (Kupchik et al., 2015) and the NAshell (Creed, Ntamati, Chandra, Lobo, & Lüscher, 2016) there is a substantial D1-MSN contribution to the indirect projection to the ventral pallidum. Thus, for the NAc, D1-MSNs contribute to both the direct as well as the indirect pathway, while akin to the rest of the striatum, D2-MSNs are restricted to the indirect pathway.
While MSNs receive input from all major neurotransmitters and neuromodulators, the acquisition of drug reward associations depends on the convergence of dopamine and glutamate release within the NAcore, and it has been shown that cue-induced relapse to drugs is mostly driven by glutamatergic inputs to the NAcore from the prelimbic cortex and basolateral amygdala (Kalivas & Volkow, 2005; Koob & Volkow, 2010; Scofield, Heinsbroek, et al., 2016; Stefanik & Kalivas, 2013). Accordingly, for the purpose of this review, we rely mainly on data regarding glutamatergic transmission and, more specifically drug-induced adaptations of glutamatergic synaptic plasticity on MSNs.
1.3. Synaptic plasticity and metaplasticity at glutamatergic synapses
Synaptic plasticity is the selective weakening and strengthening of synaptic connections in response to distinct patterns of activity, and has been proposed as the neurobiological manifestation of learning (Kandel, 2001). Glutamate is the major excitatory neurotransmitter in the brain and acts on ionotropic and metabotropic glutamate receptors to regulate neuronal activity. The main effectors of glutamatergic transmission are the ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), homo- or heterotetramers composed of four pore-forming subunits GluA1-4 (Greger, Watson, & Cull-candy, 2017). N-methyl-D-aspartic acid receptors (NMDARs) are hetero-tetramers composed of 7 differentially expressed subunits: GluN1, four GluN2 (GluN2A-D) and two GluN3 (Paoletti, Bellone, & Zhou, 2013) and are the key determinant for the expression of many different forms of postsynaptic plasticity (Malenka & Bear, 2004). Metabotropic glutamate receptors, coupled to G-proteins, link glutamate binding to downstream signaling pathways and are also implicated in the induction and expression of many different forms of synaptic plasticity (Anwyl, 2009; Atwood, Lovinger, & Mathur, 2014). A change in efficacy of glutamate transmission is most frequently attributed to a change in the probability of presynaptic neurotransmitter release (Castillo, 2012) or a change in the density (insertion or removal) and/or functionality (post-translational modification, change in subunit composition) of postsynaptic AMPAR (Malenka & Bear, 2004). Although many distinct signaling pathways can lead to the expression of synaptic plasticity, pre- and postsynaptic calcium signaling is involved in a majority. The most prominent forms of glutamatergic plasticity depend on the activation of postsynaptic NMDAR or metabotropic glutamate receptors.
Synaptic plasticity can be non-associative or depend on the orchestrated activation of the presynaptic afferent and postsynaptic neuron. Hebbian plasticity is the primary theoretical model describing the neuronal basis of associative learning, and has been demonstrated in many model systems. The general principles of Hebbian plasticity, such as input specificity, associativity and bidirectionality, can be applied to many forms of pre- and postsynaptic plasticity. The direction of plasticity depends on distinct activity patterns of pre- and postsynaptic neurons (either induced by sensory experience or by experimenter-induced stimulation patterns) with low activity (or low synchrony between pre and postsynapse) leading to long-term depression (LTD) and higher activity (or high synchrony between pre and postsynapse) leading to long-term potentiation (LTP) (Figure 1a). However, models show that networks that rely exclusively on activity dependent plasticity thresholds are unstable. Left uncontrolled they cause a feed forward, ‘runaway’, strengthening or weakening of synapses. The resulting occlusion of plasticity makes further plasticity impossible and limits information processing. The Bienenstock-Cooper-Munro theory (BCM theory) found a theoretical solution for these limitations by adding an activity dependent plasticity threshold Θ (Figure 1A) (Cooper & Bear, 2012). The implementation of the BCM theory in network models has demonstrated that the modified Θ thresholds is necessary to maintain a functional dynamic range, to increase information storage capacity and to ensure network stability (Fusi & Abbot, 2007). For example, upon strong network activity Θ; is biased towards the induction of LTD, thereby keeping synapses within a dynamic range. The success of the BCM theory led to the search of the biological implementation of adjustable plasticity thresholds within the nervous system which were subsequently discovered at different synapses in many brain nuclei (Abraham, 2008; Cooper & Bear, 2012). This form of higher order plasticity was termed metaplasticity (Abraham & Bear, 1996).
Figure 1. Synaptic Metaplasticity: Concept and mechanisms.
A) BCM theory: A sliding synaptic plasticity threshold prevents synapses from saturating. The solid blue line illustrates the change in synaptic strength as a function of afferent activity in control conditions. Low afferent activity causes LTD whereas LTP is induced upon reaching a certain threshold Θ. An overall increase in network activity shifts the synaptic plasticity threshold to the right (Θi, see arrow), which prevents saturation of the synapse. B) Metaplasticity mechanisms can affect i) how afferent activity is translated into neurotransmitter release, ii) how glutamate release is coupled to receptor activation and intracellular calcium signaling, and iii) how downstream molecules react to the calcium signal
Just as the varied mechanisms involved in synaptic plasticity, the diversity of metaplasticity mechanisms is vast (Figure 1B). Metaplasticity mechanisms can either affect i) how afferent activity is translated into neurotransmitter release, ii) how glutamate release is coupled to receptor activation and intracellular calcium signaling and iii) how downstream molecules react to this calcium signal. Hence, a change in expression and function of any signaling molecule within a synaptic plasticity pathway can induce metaplasticity. For a more detailed introduction to the many forms of metaplasticity (i.e. heterosynaptic metaplasticity, metaplasticity of inhibitory transmission) the reader is referred to a number of excellent reviews on these topics (Abraham, 2008; Abraham & Bear, 1996; Antonov, Kandel, & Hawkins, 2010; Jones, 2015).
2 Metaplasticity in drug addiction
After a protracted drug-free period, NAc neurons from cocaine-experienced animals show a potentiation of AMPAR-mediated synaptic transmission in the NAshell (Kourrich, Rothwell, Klug, & Thomas, 2007) and NAcore (Gipson, Kupchik, et al., 2013). A similar strengthening of synapses has been shown for methamphetamine (Jedynak et al., 2016), nicotine (Gipson, Reissner, et al., 2013), ethanol (Spiga et al., 2014) but not for heroin (Shen, Moussawi, Zhou, Toda, & Kalivas, 2011). Several studies indicate that withdrawal-induced strengthening of synapses could depend on cell type (D1 versus D2 MSNs) (Pascoli, Turiault, & Lüscher, 2012) and afferent input (Britt et al., 2012; MacAskill, Cassel, & Carter, 2014; Pascoli et al., 2014). Lüscher and colleagues have demonstrated in several studies that reversing this synaptic potentiation can prevent reinstated drug seeking, indicating that these drug-induced alterations of glutamatergic synaptic transmission in the NAc could harbor the cellular substrates for drug seeking and relapse vulnerability (Pascoli et al., 2014, 2012). In addition to this constitutive potentiation of synapses after drug withdrawal, exposure to cues associated with drug use elicits rapid and transient morphological and electrophysiological changes at glutamatergic synapses in the NAcore (Gipson, Kupchik, & Kalivas, 2014).
In addition to modulating the efficiency of glutamatergic transmission, drugs of abuse have been shown to affect the capacity of glutamatergic synapses to undergo plasticity. Table 1 gives as overview of the literature describing drug-induced metaplasticity at glutamatergic synapses onto MSNs in the NAc. Although the variability of drug administration protocols makes direct comparisons somewhat difficult, one common form of impaired plasticity is a loss of NMDA-dependent LTD. This LTD has a postsynaptic locus and is usually induced by a low frequency pairing protocol (LFSp-LTD 1-5 Hz stimulation + depolarization of the postsynaptic cell to -50 mV). NMDAR-dependent LTD is abolished in vitro after withdrawal from non-contingent cocaine injections (Thomas, Beurrier, Bonci, & Malenka, 2001), as well as in vitro and in vivo after withdrawal or extinction from cocaine self-administration (Kasanetz et al., 2010b; Martin, Chen, Hopf, Bowers, & Bonci, 2006; Moussawi et al., 2009), in vivo after extinction from heroin self administration (Shen et al., 2011) and in vitro after chronic ethanol exposure (Jeanes, Buske, & Morrisett, 2014; Renteria, Jeanes, & Morrisett, 2014; Spiga et al., 2014). These studies indicate that, despite the different pharmacological properties of cocaine, heroin and ethanol all drugs induce similar impairments in synaptic plasticity. However in most studies in Table 1, the description of drug-induced metaplasticity is phenomenological and the detailed underlying synaptic mechanisms remain to be revealed. Accordingly, the next section will give an overview of distinct metaplasticity substrates at the tetrapartite synapse, describe how some of these substrates are affected by the exposure to drugs of abuse allowing changes in synaptic plasticity and behavior.
Table 1. Drug induced metaplasticity at glutamatergic MSN synapses in the NAc. Presynaptic plasticity Postsynaptic plasticity.
| Drug | Treatment paradigm | Metaplasticity | Potential Mechanism | Reference |
|---|---|---|---|---|
| Cocaine | 1×IP+1dWithdrawal | Loss of eCB-LTD in NAc core MSNs | Decrease of surface mGLUR5 | (Fourgeaud et al., 2004) |
| 5×IP+Withdrawal | Reduced LFSp-LTD in NAc shell MSNs | N/A | (Thoma s et al., 2001) | |
| SA+Abstinence | 1 day abstinence: LFSp-LTD is abolished in MSN shell and core21 days abstinence: LFSp-LTD is abolished in MSN core but NOT in MSN shell | N/A | (M. Martin et al., 2006) | |
| IP | Decreased threshold for acute cocaine induced LTD | Depotentiation | (Kourrich et al., 2007) | |
| SA+Extinction | Loss of HFS-LTP impaired LFS-LTD | LTP: occlusion LTD: N/A | (Moussawi et al., 2009) | |
| SA+long access SA 1d Withdrawal | LFSp-LTD remains abolished in an ‘addicted’ subpopulation but is restored in most rats | N/A | (Kasanetz et al., 2010a) | |
| SA+Extinction | Loss of mGluR5-LTD after extinction training | mGLUR5 function | (Knackstedt, Moussawi, LaLumiere, Schwendt, & Kalivas, 2010) | |
| 1×IP+1dWithdrawal | Loss of eCB-LTD in NAc core D2 MSNs | N/A | (Grueter, Brasnjo, & Malenka, 2010) | |
| 5d IP+≥7d Withdrawal | Loss of DHPG and LFSp-LTD -LTD in MSNs of NAc shell but not core | D1R signaling | (C.-C. Huang et al., 2011) | |
| SA+≥45 Withdrawal | DHPG-LTD: Switch from mGluR5-eCB signaling to mGLUR1-CP-AMPAR signaling | Deregulation of mGluR5 | (McCutcheon et al., 2011) | |
| Loss of HFS-LTP in NAc shell D1 MSNS | Occlusion | (Pascoli et al., 2012) | ||
| SA+≥45d Withdrawal | Appearance of LFSp-LTD -LTD in BLA-NAc Shell synapses | Increase of CP-AMPAR | (B. R. Lee et al., 2013) | |
| SA+≥45d Withdrawal | Appearance LFSp-LTD -LTD in mPFC-NAc Shell synapses | Increase of CP-AMPAR | (Y.-Y. Ma et al., 2014) | |
| D-amphetami ne | Locomotor sensitization | LFSp-LTD in shell MSNs | N/A | (Brebner et al., 2005) |
| Morphine | 7d IP+ 7D withdraw al | Decreased mGluR2/3 agonist induced LTD | N/A | (Robbe, Bockaert, & Manzoni, 2002) |
| Heroin | SA+Extinction | Decreased threshold for acute heroin induced LTP | NR2B function | (Shen et al., 2011) |
| THC/ WIN55,212-2 | 7d IP+1d Withdrawal | Loss of eCB-LTD in NAc shell MSNs | CB1/MOR desensitization | (Hoffman, Oz, Caulder, & Lupica, 2003) |
| THC/WIN55,212-2 | 1d IP+1d Withdrawal | Loss of eCB-LTD NAc core MSNs | CB1 desensitization | (Mato et al., 2004) |
| Ethanol | Chronic intermittent | Switch from LFSp-LTD to LTP | Defect eCB signaling | (Renteria et al., 2014) |
| Chronic intermittent | D1 MSNs Switch from LFSp-LTD to LTP D2 MSNs Switch from no plasticity to LFSp-LTD | D1: upregulation of NMDAR D2 MSNS: downregulation of NMDAR | (Jeanes et al., 2014; Renteria, Maier, Buske, & Morrisett, 2017) | |
| Chronic | loss of LFSp-LTD in NAc shell MSNs in EtOH withdrawn animals | Dopamine levels and/or occlusion | (Spiga et al., 2014) |
2.1 Potential metaplasticity substrates and drug induced adaptations at the tetrapartite synapse
Although the vast majority of studies demonstrating either synaptic plasticity or synaptic metaplasticity use pre- and postsynaptic physiology as the experimental read-out, it has become increasingly clear that the view of synaptic physiology being determined only by the canonical pre- and postsynapse is over-simplified. Recently, a number of studies in the field of addiction have demonstrated an active role for perisynaptic astroglial processes (PAPs) and the extracellular matrix signaling domain in regulating excitatory synaptic plasticity in the NAc and other brain regions (Mulholland, Chandler, & Kalivas, 2016). This awareness has resulted in conceptualizing the functional synapse as a ‘tetrapartite synapse’, where all four components (pre- and postsynapse, astroglia and extracellular matrix) function in concert (Dityatev & Rusakov, 2011). Indeed, when considering the constitutive changes in synaptic function produced by activity that guide metaplasticity, the noncanonical components appear to play an especially important role.
2.1.1 The Presynaptic Terminal
Presynaptic long-term plasticity is manifested as a change in the magnitude and reliability of glutamate release (Castillo, 2012; Y. Yang & Calakos, 2013). It can be either induced directly via repetitive afferent stimulation or involves retrograde messenger released from the postsynaptic cell. The most prominent forms of presynaptic LTP involve the activation of adenylate cyclase (AC) and subsequent increase in cyclic adenosine monophosphate (cAMP) which activates protein Kinase A (PKA). PKA has been demonstrated to promote neurotransmitter release by directly acting on substrates in the transmitter release machinery (Castillo, Schoch, Schmitz, Südhof, & Malenka, 2002). The activation of AC can occur due to strong afferent activation, usually 100 Hz (Castillo, 2012) and can be facilitated by activation of Gs coupled presynaptic receptors (Bartsch et al., 2015; Li & Rainnie, 2014).
Many forms of presynaptic LTD involve the activation of presynaptically located Gi/o coupled G-protein coupled receptors (GPCRs) such as D2R (Calabresi et al., 1997), cannabinoid 1 receptors (CB1R) (Robbe, Kopf, Remaury, Bockaert, & Manzoni, 2002), mGluR2/3 (Robbe, Bockaert, et al., 2002) or mu-opioid receptors (MOR) (Heinsbroek et al., 2017). Upon activation of these receptors, the G-Protein dissociates into Gαi and Gβγ subunits. Both of these subunits contribute to the inhibition of neurotransmitter release (Atwood et al., 2014). The Gβγ subunits have been shown to activate K+ channels and to inhibit voltage-gated calcium channels (VGCC) (Atwood et al., 2014). These actions result in decreased excitability and reduced activity dependent Ca2+ entry into the presynaptic terminal, which decreases the probability of vesicle fusion. In addition, Gβγ might also have a direct inhibitory influence on components of the vesicular release machinery (Blackmer, 2001). The Gαi subunits inhibit AC, resulting in decreased cAMP levels.
Presynaptic adaptations and metaplasticity
While metaplasticity is well documented for postsynaptically expressed forms of long-term plasticity, metaplasticity of presynaptically expressed forms of long-term plasticity has received less attention. Many forms of presynaptic metaplasticity described seem to be related to a change in transmitter release probability (McHail & Dumas, 2015). Goussakov and colleagues have demonstrated that weeks after a status epilepticus episode, the hippocampal mossy fiber pathway exhibits increased transmitter release probability and a marked loss in presynaptic LTP, indicating occlusion of LTP at already potentiated synapses (Goussakov, Fink, Elger, & Beck, 2000). Although previous work has described that priming activation of metabotropic receptors can induce metaplasticity, most of these studies did not unequivocally examine the locus of plasticity (Bortolotto, Bashir, & Collingridge, 1994; Mellentin & Abraham, 2001).
How presynaptic release probability can affect postsynaptic plasticity
Presynaptic release probability and short-term plasticity influence the amount of glutamate released during plasticity induction. Accordingly could presynaptic release probability have direct impact on thresholds for postsynaptic plasticity, or changes in release probability drive postsynaptic metaplasticity. However, surprisingly few studies have examined the direct effect of changes in neurotransmitter release probability on postsynaptic metaplasticity. Safo and Regehr have demonstrated that the activation of presynaptic CB1R is necessary for the induction of a postsynaptically expressed LTD (Safo & Regehr, 2005). However, the authors suggested that CB1R activation induced the release of an anterograde messenger that ultimately acts on the postsynaptic cell to induce LTD. Interestingly, Safo and Regehr did not consider that presynaptic modulation of transmitter release during LTD induction could also account for the CB1R dependence of postsynaptic LTD. A very elegant study by Blundon et al has demonstrated that a “critical period” for plasticity at synapses between the thalamus and the auditory cortex is ended after the establishment of presynaptic gating by Adenosine1 Receptors (A1R) (Blundon & Zakharenko, 2013). The activation of A1R leads to a strong short-term inhibition of glutamate release during LTD induction and prevents postsynaptic LTD. The blockade of A1R does not only reestablish postsynaptic LTD but also reopens the “critical period” at thalamo-cortical synapses in adult mice and improves auditory perception (Blundon et al., 2017).
Another example of how presynaptic release probability could potentially affect postsynaptic metaplasticity comes from Lee, Yasuda & Ehlers. The authors have shown that spontaneous glutamate release can set the subunit composition of postsynaptic NMDR and drives metaplasticity at glutamatergic synapses in hippocampal neurons (M.-C. Lee, Yasuda, & Ehlers, 2010). These results indicate that presynaptic plasticity can act as a very powerful modulator of postsynaptic metaplasticity.
Drug induced presynaptic neuroadaptations and metaplasticity
It is well established that adaptations in NAc glutamatergic transmission are a core feature of addiction-related pathophysiological states (Kalivas, 2009), however our understanding of postsynaptic alternations (see section 2.1.2) exceeds those of presynaptic adaptations.
Using optogenetic tools it was demonstrated that presynaptic release probability (Pr) of NAc shell afferents was enhanced after short-term (1 d) and long-term (45 d) withdrawal from either non-contingent (i.p. injection) or contingent (self-administration) exposure to cocaine. The increase of release probability was specific for afferents from PFC and PVT; no change was found for BLA afferents indicating a procedure- and pathway-specific effect (Neumann et al., 2016; Suska, Lee, Huang, Dong, & Schluter, 2013). This increased release probability could bias the general responsiveness of the NAc shell towards PFC and PVT afferents and also favor establishment of new synapses and synapse specific potentiation for these afferents. Which adaptations in the presynapse are driving the pathway specific changes in release probability remains to be investigated. For the NAc core it has been demonstrated that decreased function of mGluR2/3 can drive metaplasticity and contributes to drug seeking behavior (Figure 2). Non contingent cocaine as well as withdrawal from nicotine and cocaine SA down-regulates presynaptic mGluR2/3 receptor function and expression (Cleva & Olive, 2012). Similarly, down-regulation of mGluR2/3 is observed in PFC neurons following chronic exposure to ethanol (Meinhardt et al., 2013). Decreased mGluR2/3 function seems to result from several different neuroadaptations such as reduced protein expression (Kasanetz et al., 2013), receptor phosphorylation (Xi et al., 2002), reduced basal glutamate tone (Moran, McFarland, Melendez, Kalivas, & Seamans, 2005) and/or up-regulated expression of activator of G protein signaling 3 (AGS3) (Bowers et al., 2004). The drug-induced impairment of this presynaptic feedback control has been demonstrated to contribute to a transient glutamate spillover during cue-induced reinstatement, which drives a transient postsynaptic potentiation of glutamatergic synapses as well as drug seeking. Decreased mGluR2/3 has been directly linked to metaplasticity induced by cocaine self-administration. The ability to induce presynaptic LTP in PFC glutamatergic afferents to the NAcore is markedly impaired in cocaine-extinguished animals compared to drug naïve animals, which arises from synaptic occlusion at already potentiated synapses. Pharmacological restoration of the basal glutamate tone on mGluR2/3 was able to reverse this drug-induced metaplasticity (Moussawi et al., 2009).
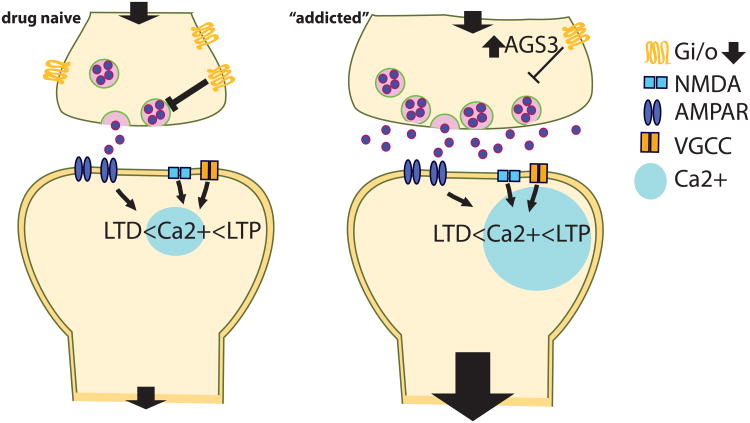
Figure 2. Presynaptic metaplasticity substrates.
Impaired presynaptic Gi/o function (i.e. mGluR2/3) at the addicted synapse (either via decreased receptor expression or up-regulation of AGS3 signaling) leads to presynaptic potentiation. Enhanced glutamate release probability causes high amounts of glutamate to be released in response to afferent activity. This enhanced excitatory drive is thought to contribute to transient synaptic potentiation during cued drug seeking, probably via decreased thresholds for LTP.
The activation of mGluR2/3 (Justinova et al., 2016; Peters & Kalivas, 2006), restoration of Cystine/Glutamate exchange (Moran et al., 2005) as well as inhibition of AGS3 (Bowers et al., 2004, 2008) has been furthermore shown to prevent reinstated drug seeking.
These results indicate that the pathway specific presynaptic alteration may interact with and contribute to other drug-induced cellular adaptations to shift the functional output of NAc neurons, contributing to the addictive emotional and motivational state.
2.1.2 The Postsynapse
Postsynaptic plasticity is manifested as a change in the density (insertion or removal) and/or functionality (phosphorylation, change in subunit composition) of postsynaptic AMPAR receptors (Malenka & Bear, 2004). Multiple forms of plasticity can be induced by activation of NMDAR, VGCCs and metabotropic glutamate receptors and the postsynaptic Ca2+ transient has been demonstrated to serve as a major instructor for the induction of postsynaptic plasticity (Holbro, Grunditz, Wiegert, & Oertner, 2010). However, how Ca2+ dynamics determine the outcome of postsynaptic plasticity is not completely understood. Common theories state that the peak calcium concentration (Lisman, 1989), the duration of the calcium elevation, as well as the location of the calcium source can determine the direction of plasticity (Evans & Blackwell, 2015). Peak calcium concentration can influence the sign of plasticity by balancing the activation of kinases and phosphatases activated by binding proteins with different affinities for Ca2+. Ca2+/calmodulin-dependent protein kinase ii (CaMKii), and calcineurin (CaN, also known as PP2B) are prominent examples of a plethora of different calcium-binding proteins involved in synaptic plasticity. CaMKii is a serine/threonine protein kinase that phosphorylates AMPARs and thereby increases their conductance but is also involved in the activity dependent exocytosis of AMPARs (Lisman, Schulman, & Cline, 2002). The protein calcineurin (CaN) is a Ca2+ -dependent serine/threonine phosphatase that is implicated in the activity dependent dephosphorylation of AMPAR as well as activation of other phosphatases (for example PPT-1) leading to AMPAR endocytosis and LTD (Collingridge, Peineau, Howland, & Wang, 2010). Interestingly, CaMKii and CaN show different affinities for Ca2+. Due to it's low affinity for Ca2+, CaMKii activation necessitates a high amplitude postsynaptic calcium signal which corresponds with the fact that induction of LTP usually needs strong and/or coincident pre and postsynaptic activity (Dan & Poo, 2004; Stanton, 1996). CaN has a higher affinity for Ca2+ and therefore it can be activated by lower neuronal activity to induce LTD (Lisman, 1989).
Besides the prevailing idea that local Ca2+ amplitude determines synaptic plasticity, the Ca2+ location within certain micro-domains in dendrites and spines could be equally important (Evans & Blackwell, 2015). Indeed it has been shown that diverse calcium sources such as NMDARs, L-type channels or internal Ca2+ stores seem to contribute to different forms of synaptic plasticity (Tigaret, Olivo, Sadowski, Ashby, & Mellor, 2016). The distinct dendritic distribution of these Ca2+ sources associated with upstream and downstream signaling molecules can create specific plasticity micro-domains on the dendritic spine (Colgan & Yasuda, 2014; Evans & Blackwell, 2015). Distinct plasticity micro-domains could be activated, depending on intensity and mode of transmitter release (i.e synaptic versus extra-synaptic, for example via astrocytes (see section 2.1.3)) and functional changes of these plasticity micro-domains could drive metaplasticity. While we cannot cover all components of this highly inter-dependent protein network, we will highlight the most prominent upstream receptors that are known to be involved in postsynaptic metaplasticity and to be modulated by exposure to drugs of abuse.
NMDAR subunit composition and metaplasticity
N-methyl-D-aspartic acid receptors (NMDARs) are hetero-tetramers composed of 7 differentially expressed subunits: GluN1, four GluN2 (GluN2A-D) and two GluN3 (Paoletti et al., 2013). The subunit composition determines the functional properties of NMDARs, such as opening kinetics and calcium conductance. The magnitude of calcium flow through open NMDAR channels is a key determinant for the expression of many different forms of postsynaptic plasticity (Malenka & Bear, 2004). Accordingly, NMDAR subunit composition and functionality are a major determinant of synaptic metaplasticity (Yashiro & Philpot, 2008). NMDAR containing the GluN2BR subunit have a higher Ca2+ conductance and thus a higher capacity to activate CaMKii, a kinase which has a low affinity for Ca2+ and is strongly implicated in activity-dependent synaptic strengthening (Herring & Nicoll, 2016). Indeed, it has been shown that functional enhancement of GluN2BR signaling can make synapses more susceptible to undergo long-term potentiation (Pérez-Otaño & Ehlers, 2005). Despite the dependence of postsynaptic LTP on NMDAR activation, a priming activation of NMDAR prior to induction can increase the threshold for LTP (Y. Y. Huang, Colino, Selig, & Malenka, 1992) and facilitate the expression of LTD without any change in basal transmission (Christie & Abraham, 1992). The mechanisms of this NMDAR priming induced metaplasticity are not completely understood, but it was suggested that it might involve a long term depression of NMDAR (Abraham, 2008). Indeed, it is now known that NMDAR function and expression is highly plastic and can be changed in an activity dependent manner (Hunt & Castillo, 2012; Kombian & Malenka, 1994; Larkman, Hannay, Stratford, & Jack, 1992; Paoletti et al., 2013; Rebola, Srikumar, & Mulle, 2010).
Drug induced changes in NMDAR subunit composition and metaplasticity
Withdrawal from nicotine (Gipson, Reissner, et al., 2013) and heroin self-administration (Shen et al., 2011) but not cocaine self-administration (Yang et al., 2017) enhances the expression of GluN2BR-containing NMDARs in the NAc core (Figure 3). Short term withdrawal from non-contingent cocaine increased GluN2BR in the NAc shell (Y. H. Huang et al., 2009). The drug-induced enrichment of NR2B is often associated with the generation of silent synapses. Silent synapses show only NMDAR- but no AMPAR-mediated currents and are therefore unclaimed substrates for synaptic plasticity (B. R. Lee & Dong, 2011). Drug induced increase in GluN2BR was suggested to depend on the functional up-regulation of CREB, since the expression of a dominant negative form of CREB prevents cocaine-induced increases in the GluN2BR/GluN2A ratio at excitatory synapses in the NAc shell and blocks motor sensitization to cocaine (Brown et al., 2011). The enrichment of GluN2BR does not occur after withdrawal from sucrose self-administration (H.-J. Yang et al., 2017), and is not dependent on contingent drug exposure (Brown et al., 2011). It has been furthermore shown that GluN2BR selective antagonists block CPP induced by morphine, but not that by natural reinforcers (Y. Y. Ma et al., 2006). Although these studies implicate that GluN2BR enrichment results from the pharmacodynamics of drug exposure and withdrawal rather than being a molecular manifestation of reward learning, the metaplasticity induced by the enrichment of GluN2BR is likely to set the stage for future relapse events. Indeed, it has been demonstrated that the NR2B selective antagonist, ifenprodil, can rescue bidirectional synaptic plasticity after cocaine self-administration (Debacker et al., 2014) as well as decrease cue-induced reinstatement of heroin (Shen et al., 2011) and nicotine (Gipson, Reissner, et al., 2013).
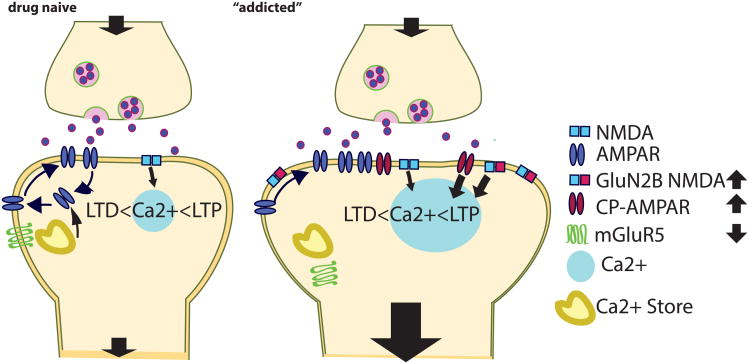
Figure 3. Postsynaptic metaplasticity substrates.
Accumulation of CP-AMPAR and NR2BR at the addicted synapse increases postsynaptic Ca2+ upon activation. The functional down-regulation and internalization of mGluR5 impairs LTD. These adaptations increase thresholds for or abolish LTD and decrease thresholds for LTP.
Calcium permeable AMPAR (CP-AMPAR)
AMPARs are homo- or heterotetramers composed of four pore-forming subunits GluA1-4 (Greger et al., 2017) and the main effectors of glutamatergic synaptic transmission. The most abundant AMPARs are heteromers containing the GluA2 subunit, which results in receptor subtypes with linear voltage response and a low Ca2+ conductance. AMPAR lacking the GluA2 subunit are highly permeable for Ca2+ and show a non-linear voltage response due to a block of the channel pore by intracellular polyamines at depolarized membrane potentials (Donevan & Rogawski, 1995).
In the adult brain, GluA2 lacking AMPAR are mainly expressed on aspiny interneurons where they have been shown to be involved in several distinct forms of synaptic plasticity (Liu & Zukin, 2007). In excitatory neurons, CP-AMPAR are relatively rare under baseline conditions, however, neuronal activity and neuronal insults can increase CP-AMPAR content. CP-AMPAR, shown to be involved in homeostatic plasticity, are dynamically up-regulated during induction and necessary for the initiation of LTP in the CA1 region of the hippocampus (Plant et al., 2006). In general, these studies suggest that the up-regulation of CP-AMPAR can modify the threshold for all forms of plasticity whose induction needs a postsynaptic rise in Ca2+ level.
Drug induced changes in AMPAR subunit composition and metaplasticity
Following prolonged abstinence (>45 days) from extended-access cocaine self-administration in adult rats, CP-AMPARs accumulate at NAc core synapses and mediate the expression of intensified “incubated” cue-induced craving (Conrad et al., 2008). Expression of CP-AMPAR on mPFC to D1-MSN terminals in the NAc core was also found after standard access cocaine self-administration in mice (Pascoli et al., 2014). A 13 Hz optogenetic stimulation protocol reversed this insertion of CP-AMPAR and inhibited cue-induced cocaine seeking. These studies demonstrate that accumulation of CP-AMPAR on NAc medium spiny neurons contributes to the behavioral drive for cue-induced drug seeking. Whether this behavior is caused by metaplasticity or by an overall enhanced excitatory drive due to the high conductance of CP-AMPAR remains to be investigated. It is, however, noteworthy that CP-AMPAR show higher conductance at hyperpolarized membrane potentials and CP-AMPAR mediated plasticity therefore does not follow hebbian rules (Laezza & Dingledine, 2011). The pathophysiological accumulation of CP-AMPAR during drug withdrawal can thereby cause metaplasticity by inverting physiological learning rules. This has been demonstrated for glutamatergic synapses of VTA neurons in vitro (Mameli, Bellone, Brown, & Lüscher, 2011) but remains to be investigated in the nucleus accumbens.
mGLUR1/5
mGluR5 and mGluR1 belong to the group 1 metabotropic glutamate receptors and are located at the perisynaptic annulus of dendrites (Katona & Freund, 2008). The activation of both receptor subtypes launches the canonic Gq signaling cascade that involves phospholipase C (PLC), PKC and the release of Ca2+ from intracellular stores but it can also activate PI3 kinase, tyrosine kinase and phosphatase, and generate endocannabinoids (Katona et al., 2006). Although mGluR1/5 signal through the same class of G proteins and are often co-expressed on the same cells, they can also have different functional roles (Poisik, Mannaioni, Traynelis, Smith, & Conn, 2003).
The positive interaction of mGluR5 with NMDAR (Pisani et al., 2001) suggests its role in synaptic plasticity and learning. Indeed mGluR1/5 activation is known to induce LTD or LTP in many different brain areas (Anwyl, 2009; Bellone, Lüscher, & Mameli, 2008) and to enhance extinction learning (Cleva et al., 2011), whereas mGluR5 antagonism impairs spatial learning, and contextual fear conditioning (Simonyi, Schachtman, & Christoffersen, 2010).
mGluR1/5 are also mediators of metaplasticity. It has been shown that a priming activation of group I mGluRs with the selective group I agonist 3,5-dihydroxyphenylglycine (DHPG) can decrease the threshold for LTP (Cohen, Raymond, & Abraham, 1998) or cause a leftward shift of the frequency-response function at CA1 synapses favoring LTP over LTD (van Dam et al., 2004). Diverse mechanisms for this metaplasticity have been proposed, such as potentiation of NMDAR (Macdonald, Jackson, & Beazely, 2007) or the increase of postsynaptic excitability (Cohen, Coussens, Raymond, & Abraham, 1999).
The membrane localization of mGluR1/5 and its coupling with downstream effectors is promoted by crosslinking via the long isoforms of the adaptor protein Homer 1b/c (Kammermeier & Worley, 2007; Park et al., 2013). Homer 1a is a short isoform that acts as a dominant negative to uncouple C-C homer isoforms from mGluR5. The induction of Homer 1a expression by salient stimuli can act as a switch between different forms of mGluR5 mediated signaling (Park et al., 2013; Roloff, Anderson, Martemyanov, & Thayer, 2010). While the expression of Homer1a alone induces LTD, H1a expression in combination with mGluR5 phosphorylation leads to a conformational change of mGluR5 and potentiation of NMDAR and LTP (Marton, Shuler, & Worley, 2015). The level of expression of different Homer isoforms therefore not only regulates the membrane expression and localization of mGluR1/5, but also the plasticity pathways in which the receptors can engage upon activation.
Drug induced changes in mGluR1/5 and metaplasticity
The NAc is one of the brain areas with the highest expression of mGluR5 (Abe et al., 1992), suggesting the involvement of mGluR5 signaling in reward learning. Accordingly mGluR5 knock-out mice show deficits in the acquisition of cocaine self-administration (Chiamulera et al., 2001). Likewise the mGluR5 receptor antagonists MPEP and MTEP reduce self-administration of addictive drugs such as cocaine and nicotine and block cue induced reinstatement (Mihov & Hasler, 2016; A. C. W. Smith et al., 2017). Positive allosteric modulation of mGluR5 receptors facilitates extinction of cocaine contextual memory (Cleva et al., 2011). A single exposure to cocaine or THC can reduce mGluR5 expression in the NAC core and PFC and abolish LTD of glutamate release (Fourgeaud et al., 2004; Mato et al., 2004). mGluR5 dependent LTD at PFC to NAc synapses is abolished in vivo in cocaine extinguished rats and rescued using longer LTD protocols, indicating an increase in LTD threshold (Moussawi et al., 2009). A reduction of mGluR5 function has also been suggested after withdrawal from long access cocaine SA (McCutcheon et al., 2011). The authors found that while DHPG drives a CB1R dependent presynaptic LTD in saline controls, it can reverse the accumulation of CP-AMPAR in the nucleus accumbens after extended access cocaine SA (McCutcheon et al., 2011). Interestingly, the DHPG induced LTD in the saline group was mGluR5 mediated, whereas DHPG-LTD in the cocaine group was mGluR1 mediated. Because the authors found no defect in CB1R function, they suggested that the impairment of mGluR5 causes the metaplastic transition from mGluR5 to mGluR1 signaling and thereby is profoundly altered (McCutcheon et al., 2011). The changed function and expression of mGluR1/5 might be caused by a drug-induced change in phosphorylation of mGluR1/5 residues by protein kinases that regulate trafficking, subcellular/subsynaptic distribution, and function. An alternative explanation could be a deregulation of Homer proteins. Extinction training from cocaine self-administration or non-contingent exposure to cocaine increases Homer 1b/c levels in the NAc and inhibits LTD by decreasing mGluR5 surface expression (Fourgeaud et al., 2004; Knackstedt, Moussawi, et al., 2010). This signaling cascade has furthermore been proposed as the synaptic basis of locomotor sensitization to cocaine (Park et al., 2013).
These studies highlight the multidimensional involvement of mGluR1/5 in drug induced changes of synaptic plasticity at different stages of drug abuse and indicate that targeting this receptor class is a promising but also challenging therapeutic avenue for the treatment of addiction. Which type of mGluR1/5 based treatment could be successful might depend on the drug abuse history of individual patients (i.e. mode of exposure, type and duration of withdrawal).
2.1.3 Astroglia
Astrocytes are a subset of glial cells that outnumber neurons in the human brain (Nedergaard, Ransom, & Goldman, 2003). Regardless of their high abundance, for a long time they were simply considered as a passive scaffold, the glue of the brain. Only recently have we started to understand that astrocytes actively contribute to synaptic signaling not only by providing metabolic support but also by agonist induced, activity dependent release of gliotransmitters such as glutamate (Araque, Li, Doyle, & Haydon, 2000; Bezzi et al., 2004), ATP (Gordon et al., 2005), and D-Serine (Schell, Molliver, & Snyder, 1995). Glutamate release from astrocytes can occur via different mechanisms such as Ca2+ and SNARE dependent vesicular release (Araque et al., 2000), glutamate exchange via the Na2+ dependent cystine-glutamate antiporter and reverse exchange via GLT-1 (Malarkey & Parpura, 2008). The astrocytic release of glutamate and D-Serine is thought to modulate NMDAR function and thus the ability to induce plasticity or set plasticity thresholds (Han et al., 2012; Panatier, Theodosis, Mothet, Touquet, & Pollegioni, 2006). Astrocytic glutamate release close to presynaptic terminals has furthermore been shown to modulate presynaptic release probability (Gomez-Gonzalo et al., 2015; R. Martin, Bajo-Graneras, Moratalla, Perea, & Araque, 2015), which is also likely to modulate metaplasticity (see section 3.1).
Astrocytes are also pivotal in maintaining synaptic microenvironments via perisynaptic processes that ensheath synapses (perisynaptic astroglial protrusions; PAPs), and restrict the diffusion of neuro- and gliotransmitters (Piet, Vargova, Sykova, Poulain, & Oliet, 2004; Verkhratsky & Nedergaard, 2014). These diffusion barriers are not static but can undergo activity dependent restructuring that controls the abundance of transmitters and co-transmitters in the synaptic vicinity (Haber, Zhou, & Murai, 2006; Oliet, Piet, & Poulain, 2001; Perez-alvarez, Navarrete, Covelo, Martin, & Araque, 2014) and has been proposed as a mechanism for metaplasticity (Jones, 2015).
Astrocytes are also the predominant location of GLT-1, a glutamate transporter that accounts for 95% of the glutamate uptake activity in the brain and tightly regulates the diffusion of glutamate (Danbolt, Furness, & Zhou, 2016). Glutamate uptake via GLT-1 allows for a focused local glutamate transmission and prevents system over-activation and excitotoxicity. Neuronal activity can rapidly and reversibly slow glutamate clearance via modulation of astrocytic GLT1-dependent glutamate transport (Armbruster, Hanson, & Dulla, 2016) but also induce long lasting changes in GLT1 function (Cheung, Sibille, Zapata, & Rouach, 2015). Since the regulation of glutamate uptake correlates so tightly with the occurrence of glutamatergic synaptic plasticity, it has been suggested to be an integral component of plasticity (Pita-Almenar, Sol Collado, Colbert, & Eskin, 2006) and metaplasticity (Abraham, 2017; Jones, 2015) at glutamatergic synapses.
Drug induced adaptations of astrocytic metaplasticity substrates
The morphology and function of astrocytes have been shown to be markedly affected by exposure to drugs of abuse and these adaptations are thought to contribute to drug induced synaptic adaptations and metaplasticity and by that to the neurobiological basis of addiction (Miguel-Hidalgo, 2009; A. C. W. Smith, Scofield, & Kalivas, 2015) (see Figure 4).
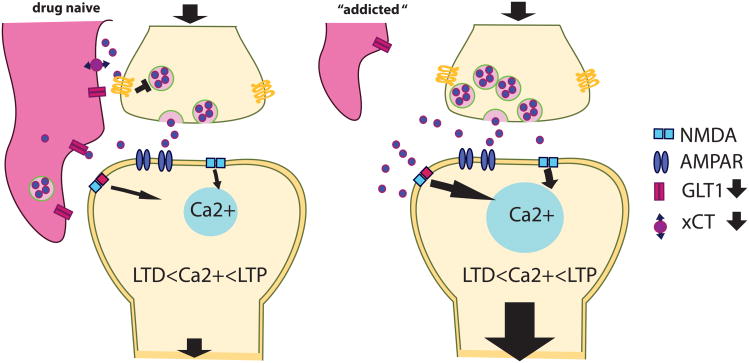
Figure 4. Astroglial metaplasticity substrates.
The down-regulation of the glutamate antiporter xCT and the glutamate transporter GLT-1 results in decreased extracellular glutamate tone and glutamate spillover in response to afferent stimulation. The synaptic microenvironment is furthermore disrupted by a retraction of perisynaptic astroglial protrusions.
Chronic exposure to both cocaine and nicotine reduces cysteine glutamate antiporter Xct expression on astrocytes, which results in a reduced basal extracellular glutamate tone acting on presynaptic mGluR2/3 autoreceptors in the nucleus accumbens (Moran et al., 2005). The resulting decrease in mGluR2/3 dependent autoinhibition has been proposed as a mechanism for the exacerbated cue induced glutamate release into the nucleus accumbens core during drug relapse (Kalivas, 2009) (see Section 2.1.1).
Another major drug induced glioadaptation in the nucleus accumbens is the down-regulation of GLT-1, which was reported after extinction from cocaine (Knackstedt, Melendez, & Kalivas, 2010), heroin (Shen, Scofield, Boger, Hensley, & Kalivas, 2014), nicotine (Gipson, Reissner, et al., 2013) as well as for the striatum and hippocampus after exposure to ethanol and metamphetamine (Alshehri, Althobaiti, & Sari, 2017). The compromised glutamate clearance due to the down-regulation of GLT-1 is possibly further aggravated by a decreased association of PAPs with NAcore synapses, as has been demonstrated in rats extinguished from cocaine self-administration (Scofield, Li, et al., 2016). Taken together, all these drug-induced adaptations in astrocytes contribute to the dysregulation of glutamate homeostasis in the nucleus accumbens. These astroglial adaptations drive a constitutive synaptic potentiation in the nucleus accumbens core (Moussawi et al., 2011) as well as enhance spillover of glutamate upon vigorous afferent activation, as was demonstrated to occur during cue induced reinstatement to heroin, cocaine and nicotine (Gipson, Reissner, et al., 2013; LaLumiere & Kalivas, 2008; A. C. W. Smith et al., 2017). The subsequent activation of extra-, peri- and even heterosynaptically located receptors such as GluN2BR or mGluR5 has been demonstrated to enable cue-induced transient synaptic potentiation and drug-seeking behavior (Gipson et al., 2014).
Interestingly, both GLT-1 down-regulation (Reissner et al., 2015), cocaine withdrawal induced synaptic potentiation (Moussawi et al., 2011) as well as retraction of PAPs can be reversed by treatment with the β-lactam antibiotic ceftriaxone (Scofield, Li, et al., 2016), and the rescue of these astrocyte functions also inhibits cue induced reinstatement (Knackstedt, Melendez, et al., 2010). Ceftriaxone does also restore LTP and LTD in cocaine extinguished rats (Moussawi et al., 2009). The direct involvement of astrocytes in the control of cue induced drug seeking has been demonstrated in a study with Gq DREADDS (designer receptors exclusively activated by designer drugs) selectively expressed in astrocytes using a cell type specific adeno virus approach (Scofield et al., 2015). Activation of these Gq DREADDs and hence depolarization of astrocytes inhibited cue induced reinstatement (Scofield et al., 2015).
2.1.4 Extracellular Matrix
The brain extracellular matrix (ECM) is a complex assembly of glycosaminoglycan (i.e. hyaluronic acid, chondroitin sulfate), proteoglycan (i.e aggrecan, versican, neurocan, brevican), glycoproteins and secreted proteins (tenascin, fibronectin, laminin, thrombospondin and reelin) (Dityatev & Schachner, 2003). The ECM serves as a structural scaffold, contributes to brain development such as cortical layer organization (Frotscher, 2010) and acts as a physical barrier that confines receptor mobility (Frischknecht et al., 2009). The ECM undergoes significant changes in structure and composition during neuronal development (Gundelfinger, Frischknecht, Choquet, & Heine, 2010). Originally it was thought that the adult ECM is less permissive for structural changes and synaptic plasticity, but recent studies have shown that experience dependent activation of matrix-shaping enzymes such as proteases and hyaluronidases can remodel the adult ECM, drives synaptic plasticity and change behavior. The regulated remodeling of the ECM exposes binding sites for cell surface receptors to orchestrate neuronal proliferation as well as structural and functional synaptic potentiation (Dityatev, Schachner, & Sonderegger, 2010). For instance the fibronectin interaction with integrin receptors facilitates the induction of LTP via SFK dependent phosphorylation of NMDAR and thus increase Ca2+ conductance (Dityatev et al., 2010). Integrin receptor activation has furthermore been shown to induce the actin polymerization needed for the enlargement of dendritic spines (Wang et al., 2008). One prominent class of ECM-shaping enzymes are a class of Zn2+-dependent endopeptidases, the so called matrix metalloproteinases (MMPs) (Huntley, 2012). Historically, MMPs were investigated for their roles in tumor cell invasion, early neurogenesis and injury triggered circuit remodeling (Huntley, 2012; A. C. W. Smith et al., 2015). Now it is known that brain derived MMPs are synthesized and secreted by neurons and glia and their localized and rapid activation drives plasticity and synaptic circuit remodeling (Allen et al., 2016; Nagy, Bozdagi, & Huntley, 2007; Wang et al., 2008). The most studied MMPs in the brain are MMP-2 and MMP-9. MMP-9 especially has recently emerged as important regulator of glutamatergic plasticity: MMP-9 activity in the hippocampus is increased upon LTP induction (Huntley, 2012) and it's activity is necessary for the late phase of LTP (Nagy et al., 2006). The facilitation of LTP could be achieved by an MMP induced enhancement of NMDAR signaling, as MMP-9 has been shown to control NMDA receptor surface diffusion through integrin beta1 signaling (Michaluk et al., 2009).
Drug induced adaptations of the ECM and metaplasticity
Evidence is emerging that some forms of plasticity of excitatory synapses that are induced by exposure, withdrawal and relapse to drugs might necessitate the reorganization of the ECM (A. C. W. Smith et al., 2015). It has been shown that activity of MMP-9 is increased in the mPFC after CPP reinstatement to cocaine (Brown, Forquer, Harding, Wright, & Sorg, 2008) and that reinstatement can be blocked by a broad spectrum MMP inhibitor (Brown et al., 2007). In a self-administration reinstatement paradigm Smith et al found enduring increased accumbal MMP-2 activity in rats after withdrawal from self-administered cocaine and transient increases in MMP-9 during cue-induced cocaine relapse. This increased MMP activity is required for both cocaine relapse and relapse-associated structural and functional transient synaptic potentiation of glutamatergic synapses in the nucleus accumbens core (A. C. W. Smith et al., 2014). And although MMP-9 knockout mice consume the same amounts of alcohol, they show impaired alcohol seeking during a motivation test and do not express the same alcohol withdrawal induced changes in synaptic plasticity in the central amygdala as their wild-type littermates (Stefaniuk et al., 2017). All these studies highlight the relevance of ECM remodeling for changes in synaptic plasticity along various stages of drug abuse, however the downstream signaling pathways that lead to metaplasticity, synapse remodeling and changed behavioral outcomes remain largely unknown and open an important direction of future research.
3. Clinical relevance: Metaplasticity as a treatment strategy for addiction
For several clinical conditions like motor impairments after stroke (Takeuchi & Izumi, 2015) or Alzheimer's disease (Jang & Chung, 2016) it has been demonstrated that increased thresholds of synaptic plasticity are contributing to the full blown clinical picture. Targeting these synaptic deficits with “correctional metaplasticity” is currently investigated as therapeutic avenue to support behavioral therapies and rehabilitation.
In the following section we describe which current treatment strategies are used to target addiction and propose how they could be improved by taking aberrant metaplasticity into account.
3.1 Pharmacotherapies - Targeting glutamate signaling at the tetrapartite synapse
Since many studies have demonstrated that the disruption of glutamate homeostasis as well as function of ionotropic and metabotropic receptors in the corticostriatal system are disrupted in addiction models, targeting the glutamatergic system is a major avenue to pharmacotherapies for drug addiction. Since preclinical studies have demonstrated the augmentation of NMDAR subunit composition and function in many animal models of drug abuse (see section 3.1.2), the NMDAR were targeted with antagonists like Memantine, Ketamine or ifenprodil in several clinical trials and have shown mixed results for the treatment of diverse classes of substance use disorder (Scofield, Heinsbroek, et al., 2016; Tomek, LaCrosse, Nemirovsky, & Olive, 2013).
As we have learned in section 3.1.3, there is ample of pre-clinical evidence on the downregulation of GLT1 on diverse pre-clinical addiction models and it has been shown that rescue of GLT1 function can restore plasticity thresholds and prevent relapse (Alshehri et al., 2017; Knackstedt, Melendez, et al., 2010; Moussawi et al., 2009; Reissner et al., 2015; Shen et al., 2014). Therefor another therapeutic approach aims to reestablish GLT1 function and glutamate homeostasis. In particular, two compounds have been studied extensively in this regard, N-acetylcysteine (NAC) and ceftriaxone, however so far only NAC was tested in clinical trials with mixed but promising results (Roberts-Wolfe & Kalivas, 2015; Spencer & Kalivas, 2017). Although preclinical studies have demonstrated the strong implication of mGlur1/5 and mGluR2/3 function in neuradaptations after exposure to drugs of abuse as well as in cue induced drug seeking, there are currently no clinical trials targeting this receptor group for the treatment of drug addiction.
Although overall these clinical trials targeting the glutamate system allow some optimism, the high outcome variability might pose problems for the translational advancement from bench to bedside. One major problem could be the ubiquitous expression and diverse function of glutamate receptors and transporters. Since the available approaches of drug application do not allow for the targeting of specific brain areas or cell populations, all the pharmacotherapies suggested so far certainly raise concern about interfering off target effects. This problem could partially be tackled by the use of positive or negative allosteric modulators (PAMs and NAMs). Allosteric modulators usually act by modulating the affinity of ligands and in that way preserve the spatial and temporal activity pattern of the receptors (Foster & Conn, 2017). Another advantage is that since allosteric modulators typically bind to sites that are less conserved than the orthosteric neurotransmitter binding sites, drug development can achieve much higher receptor selectivity with allosteric modulators than with classic agonists or antagonist (Foster & Conn, 2017). Positive allosteric modulators of glutamate signaling could help to support behavioral therapies such as cue exposure therapy (CET) that aims to reduce the strong reactivity to drug associated cues by exposing abstinent drug users to these cues while preventing drug use.
Although CET is an established and successful approach for the treatment of phobias, anxiety disorders and posttraumatic stress disorder, there is currently no consistent evidence for the efficacy of cue-exposure in treatment of addiction (Conklin & Tiffany, 2002). It has been suggested, that CET could be boosted by combination with cognitive enhancers that could lower the threshold for synaptic plasticity. A well-studied molecule in this regard is the NMDAR co-agonist D-cycloserine (DCS). DCS has been demonstrated to enhance extinction of drug associated CPP and SA in rodent models, and exposure therapy for divers phobias and social anxiety and indeed, there is some clinical evidence of efficacy of DCS in exposure therapy for nicotine and cocaine addiction (Myers, Carlezon, & Davis, 2011). As a co-agonist of NMDAR, DCS does not directly affect synaptic strength but can modulate the induction of synaptic plasticity. Interestingly it has been shown that DCS can reverse the stress-induced suppression of LTP (Richter-Levin & Maroun, 2010), indicating that the positive effect on CET could be driven by metaplasticity.
The administration of PAMs for mGluR5 restores cocaine induced impairments in LTD (Knackstedt, Trantham-Davidson, & Schwendt, 2014), facilitate extinction of cocaine CPP, ethanol SA and cocaine SA (Cleva et al., 2011; Gass et al., 2014; Gass & Olive, 2009) and decreases reinstatement in a preclinical model of CET (Perry, Reed, Zbukvic, Kim, & Lawrence, 2016). Given these positive preclinical trials the combination of CDPPB with CET could be a promising approach to increase the outcome of CET for substance use disorders.
3.2 non-invasive brain stimulation
Non-invasive brain stimulation (NIBS) techniques are quickly translating from research into clinical practice and they have recently drawn a lot of public attention as very promising therapeutic interventions for addiction.
There are two main approaches that differ in the stimulation techniques used: transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS). tDCS uses two scalp electrodes, usually 3-7 cm apart to apply direct current stimulation in the 1 mA range to the brain. tDCS is thought to modulate the excitability within the target brain region and it reaches a spatial resolution of around 1 cm, however focal stimulation becomes more difficult for deeper structures (Dunlop, Hanlon, & Downar, 2017; Nitsche et al., 2008). rTMS uses a handheld induction coil placed against the scalp to apply magnetic field pulses. rTMS is thought to modulate synaptic strength within the target brain region, with the direction of the change depending on intensity duration and stimulation pattern (Dunlop et al., 2017). rTMS is already approved for the treatment of major depressive disorders in the United states, Europe and Canada and is currently in clinical trials for effectiveness in the treatment of substance use disorders. Most clinical trials so far have targeted the “cold” executive control circuits by application of dorsolateral prefrontal cortex (dlPFC) stimulation in cocaine, nicotine and alcohol addicts and aimed to decrease craving. The outcome of these trials is variable, with about half of the studies showing a main effect of rTMS on craving, whereas the other half showed no effect (Hanlon et al., 2015). A recent study by Hanlon et al for the first time targeted the “hot” limbic circuitry by medial prefrontal/frontal pole stimulation (Hanlon et al., 2015). Continuous theta burst stimulation over the MPFC leads to decreased activity in the prefrontal cortex, nucleus accumbens and anterior insular and decreases cocaine craving (Hanlon et al., 2015). These results demonstrate that NIBS (so far mainly with rTMS) holds promising potential for the treatment of substance abuse disorders. In general, NIBS have been reported to have a high compatibility; the most frequently describe side but rarely occurring side effect are seizures. However, the global strengthening and weakening of synaptic connections within neuronal networks might also have off target effects that have not been thoroughly investigated yet (Wassermann, 2000). Another major drawback of NIBS is the huge variability of effect (Dunlop et al., 2017; Hanlon et al., 2015). To find the best stimulation parameters and experimental design to reduce this variability will be one of the big challenges for therapy development. One caveat could be that although NIBS allows for a pathway specific modification of brain circuits, which makes this approach somewhat more precise than a pure pharmacological approach, it does not allow to selectively target the specific cell ensembles that have been demonstrated to carry drug associated memories (Bobadilla et al., 2017). The interference with other emotional memories could be one explanation for the highly variably outcomes of rTMS. An alternative strategy would be to use subthreshold stimulations that do not change synaptic strength but induce metaplasticity to prime brain areas or cell ensembles that become subsequently engaged in a behavioral task. This approach is already used in motor rehabilitation therapies for stroke patients, capitalizing on metaplasticity to enhance the effects of behavioral therapy. rTMS is used to prime the motor cortex to make subsequent behavioral therapy more effective (Cassidy, Gillick, & Carey, 2014). In that regard it would be very interesting to see whether a combination of NIBS and CET could be used to improve the outcome of therapies for substance use disorders.
4. Conclusions
In this review we aimed to give an overview on the characteristics of synaptic metaplasticity in preclinical addiction models. We illustrated the general concept of homosynaptic plasticity and metaplasticity and described which drug induced adaptations at the tetrapartite glutamatergic synapse can lead to metaplasticity. An extensive list of preclinical publications has demonstrated that metaplasticity is a common denominator of drug-induced neuroadaptations, across multiple classes of addictive drugs. Therefore, we propose that the investigation of the underlying mechanism of drug-induced metaplasticity will be an essential avenue for future addiction research. Targeting drug-induced metaplasticity to reset the capacity for synaptic plasticity in combination with behavioral therapies for substance use disorders could be a promising treatment strategy for the future.
Highlights.
Metaplasticity is a higher order plasticity, which is not expressed as a change in synaptic strength but as a change in the threshold or rules to induce plasticity.
Chronic drug abuse can lead to adaptations at the tetrapartite synapses can change plasticity rules and induce metaplasticity in preclinical models.
Targeting these synaptic adaptations can restore physiological plasticity and inhibit drug seeking.
“correctional metaplasticity”, i.e. the restoration of physiological plasticity thresholds could lead to more efficient addiction therapies.
Acknowledgments
This work was supported by grants DA003906 and DA012513 (PWK).
Abbrivetions
- LTD
Long term depression
- LTP
Long term potentiation
- NAc
Nucleus accumbens
- CPP
Conditioned place preference
- SA
Self-administration
- PFC
Prefrontal Cortex
- BLA
basolateral amygdala
- MSN
Medium spiny Neuron
- BCM theory
Bienenstock-Cooper-Munro theory
- LFSp
low frequency pairing protocol
- PK
protein kinase
- cAMP
cyclic adenosine monophosphate
- AC
adenylate cyclase
- CaMKii
Ca2+/calmodulin-dependent protein kinase ii
- AGS3
activator of G protein signaling 3
- MMP
matrix metalloproteinase
- NIBS
Non-invasive brain stimulation
- tDCS
transcranial direct current stimulation
- rTMS
transcranial magnetic stimulation
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+signal transduction. Journal of Biological Chemistry. 1992;19(19):13361–13368. [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nature Reviews Neuroscience. 2008;9(5):387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Astrocytes and synaptic plasticity in health and disease. Experimental Brain Research. 2017;235(6):1645–1655. doi: 10.1007/s00221-017-4928-1. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity : plasticity of synaptic plasticity. Trends in Neurosciences. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Allen M, Ghosh S, Ahern GP, Villapol S, Maguire-zeiss KA, Conant K. Protease induced plasticity : matrix metalloproteinase-1 promotes neurostructural changes through activation of protease activated receptor 1. Scientific Reports. 2016;6:35497(October):1–17. doi: 10.1038/srep35497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri FS, Althobaiti YS, Sari Y. Effects of Administered Ethanol and Methamphetamine on Glial Glutamate Transporters in Rat Striatum and Hippocampus. Journal of Molecular Neurosciene. 2017;61:343–350. doi: 10.1007/s12031-016-0859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov I, Kandel ER, Hawkins RD. Presynaptic and Postsynaptic Mechanisms of Synaptic Plasticity and Metaplasticity during Intermediate-Term Memory Formation in Aplysia. Journal of Neuroscience. 2010;30(16):5781–5791. doi: 10.1523/JNEUROSCI.4947-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56(4):735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE Protein-Dependent Glutamate Release from Astrocytes. Journal of Neuroscience. 2000;20(2):666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster M, Hanson E, Dulla CG. Glutamate Clearance Is Locally Modulated by Presynaptic Neuronal Activity in the Cerebral Cortex. Journal of Neuroscience. 2016;36(40):10404–10415. doi: 10.1523/JNEUROSCI.2066-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lovinger DM, Mathur BN. Presynaptic long-term depression mediated by Gi/o-coupled receptors. Trends in Neurosciences. 2014;37(11):663–673. doi: 10.1016/j.tins.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch JC, Fidzinski P, Huck JH, Hörtnagl H, Kovács R, Liotta A, … Behr J. Enhanced Dopamine-Dependent Hippocampal Plasticity after Single MK-801 Application. Neuropsychopharmacology. 2015;40(4):987–995. doi: 10.1038/npp.2014.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Lüscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cellular and Molecular Life Sciences. 2008;65(18):2913–2923. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nature Neuroscience. 2004;7(6):613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Blackmer T. G Protein beta gamma Subunit-Mediated Presynaptic Inhibition: Regulation of Exocytotic Fusion Downstream of Ca2+ Entry. Science. 2001;292(5515):293–297. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- Blundon JA, Roy NC, Teubner BJW, Yu J, Eom T, Sample KJ, et al. Zakharenko SS. Restoring auditory cortex plasticity in adult mice by restricting thalamic adenosine signaling. Science. 2017;356(6345):1352–1356. doi: 10.1126/science.aaf4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundon JA, Zakharenko SS. Presynaptic Gating of Postsynaptic Synaptic Plasticity. The Neuroscientist. 2013;19(5):465–478. doi: 10.1177/1073858413482983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobadilla AC, Heinsbroek JA, Gipson CD, Griffin WC, Fowler CD, Kenny PJ, Kalivas PW. Corticostriatal plasticity, neuronal ensembles, and regulation of drug-seeking behavior. Brain Research in Addiction. 2017 doi: 10.1016/bs.pbr.2017.07.013. [DOI] [PMC free article] [PubMed]
- Bortolotto ZA, Bashir ZI, Collingridge GL. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994;368:740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Hopf FW, Chou JK, Guillory AM, Chang SJ, Janak PH, et al. Diamond I. Nucleus accumbens AGS3 expression drives ethanol seeking through G betagamma. Proc Natl Acad Sci U S A. 2008;105(34):12533–12538. doi: 10.1073/pnas.0706999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, et al. Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42(2):269–81. doi: 10.1016/s0896-6273(04)00159-x. Retrieved from http://doi.wiley.com/10.1002/ana.22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, et al. Wang YT. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310(5752):1340–3. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt Ra, Stuber GD, Wise Ra, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76(4):790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learning & Memory. 2007;14(3):214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Harding JW, Wright JW, Sorg BA. Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse. 2008;62(12):886–889. doi: 10.1002/syn.20562. [DOI] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, et al. Schluter OM. A Silent Synapse-Based Mechanism for Cocaine-Induced Locomotor Sensitization. Journal of Neuroscience. 2011;31(22):8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, et al. Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Reports. 2012;2(1):33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, et al. Borrelli E. Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1997;17(12):4536–44. doi: 10.1523/JNEUROSCI.17-12-04536.1997. Retrieved from http://www.jneurosci.org/content/17/12/4536.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, et al. Nestler EJ. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(10):2726–2731. doi: 10.1073/pnas.1521238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy JM, Gillick BT, Carey JR. Priming the Brain to Capitalize on Metaplasticity in Stroke Rehabilitation. Physical Therapy. 2014;194(1):139–150. doi: 10.2522/ptj.20130027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE. Presynaptic LTP and LTD of Excitatory and Inhibitory Synapses. Cold Spring Harbor Perspectives in Biology. 2012;4(2) doi: 10.1101/cshperspect.a005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Südhof TC, Malenka RC. RIM1α is required for presynaptic long-term potentiation. Nature. 2002;415(6869):327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Cheung G, Sibille J, Zapata J, Rouach N. Activity-dependent plasticity of astroglial potassium and glutamate clearance. Neural Plasticity. 2015;2015 doi: 10.1155/2015/109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, et al. Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4(9):873–874. doi: 10.1038/nn0901-873. Retrieved from . [DOI] [PubMed] [Google Scholar]
- Christie BR, Abraham WC. Priming of associative long-term depression in the dentate gyrus by theta frequency synaptic activity. Neuron. 1992;9(1):79–84. doi: 10.1016/0896-6273(92)90222-y. [DOI] [PubMed] [Google Scholar]
- Cleva RM, Hicks MP, Gass JT, Wischerath KC, Plasters ET, Widholm JJ, Olive MF. mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration. Behavioral Neuroscience. 2011;125(1):10–9. doi: 10.1037/a0022339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleva RM, Olive MF. mGlu receptors and drug addiction. Wiley Interdisciplinary Reviews. Membrane Transport and Signaling. 2012;1(3):281–295. doi: 10.1002/wmts.18. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3364533&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Coussens CM, Raymond CR, Abraham WC. Long-lasting increase in cellular excitability associated with the priming of LTP induction in rat hippocampus. Journal of Neurophysiology. 1999;82(6):3139–48. doi: 10.1152/jn.1999.82.6.3139. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10601447. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Raymond CR, Abraham WC. Priming of Long-Term Potentiation Induced by Activation of Metabotropic Glutamate Receptors Coupled to Phospholipase C. Hippocampus. 1998;170:160–170. doi: 10.1002/(SICI)1098-1063(1998)8:2<160::AID-HIPO8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Colgan LA, Yasuda R. Plasticity of Dendritic Spines: Subcompartmentalization of Signaling. Annual Review of Physiology. 2014;76(1):365–385. doi: 10.1146/annurev-physiol-021113-170400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nature Reviews Neuroscience. 2010;11(7):459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–21. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LN, Bear MF. The BCM theory of synapse modification at 30: interaction of theory with experiment. Nature Reviews Neuroscience. 2012;13(11):798–810. doi: 10.1038/nrn3353. [DOI] [PubMed] [Google Scholar]
- Creed M, Ntamati NR, Chandra R, Lobo MK, Lüscher C. Convergence of Reinforcing and Anhedonic Cocaine Effects in the Ventral Pallidum. Neuron. 2016:214–226. doi: 10.1016/j.neuron.2016.09.001. [DOI] [PMC free article] [PubMed]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44(1):23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Furness DN, Zhou Y. Neuronal vs glial glutamate uptake: Resolving the conundrum. Neurochemistry International. 2016;98:29–45. doi: 10.1016/j.neuint.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Debacker J, Hawken ER, Normandeau CP, Jones Aa, Di Prospero C, Mechefske E, et al. Dumont C. GluN2B-Containing NMDA Receptors Blockade Rescues Bidirectional Synaptic Plasticity in the Bed Nucleus of the Stria Terminalis of Cocaine Self-Administering Rats. Neuropsychopharmacology. 2014;40(10):394–405. doi: 10.1038/npp.2014.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Current Opinion in Neurobiology. 2011;21(2):353–359. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Schachner M. Extracellular Matrix Molecules and Synaptic Plasticity. Nature Reviews Neuroscience. 2003;4(June):456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nature Reviews Neuroscience. 2010;11(11):735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(20):9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop K, Hanlon CA, Downar J. Noninvasive brain stimulation treatments for addiction and major depression. Annals of the New York Academy of Sciences. 2017;1394(1):31–54. doi: 10.1111/nyas.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RC, Blackwell KT. Calcium: Amplitude, Duration, or Location? The Biological Bulletin. 2015;228(1):75–83. doi: 10.1086/BBLv228n1p75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB. The Nucleus Accumbens: An Interface Between Cognition, Emotion, and Action. Annual Review of Psychology. 2015;66(1):25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Conn PJ. Review Allosteric Modulation of GPCRs : New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron. 2017;94(3):431–446. doi: 10.1016/j.neuron.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hémar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2004;24(31):6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nature Neuroscience. 2009;12(7):897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- Frotscher M. Role for Reelin in stabilizing cortical architecture. Trends in Neurosciences. 2010;33(9):407–414. doi: 10.1016/j.tins.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Fusi S, Abbot L. Limits on the memory storage capacity of bounded synapses. Nature Neuroscience. 2007;10(4):485–493. doi: 10.1038/nn1859. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009;65(8):717–720. doi: 10.1016/j.biopsych.2008.11.001.Positive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Trantham-Davidson H, Kassab AS, Glen WB, Olive MF, Chandler LJ. Enhancement of Extinction Learning Attenuates Ethanol-Seeking Behavior and Alters Plasticity in the Prefrontal Cortex. The Journal of Neuroscience. 2014;34(22):7562 LP–7574. doi: 10.1523/JNEUROSCI.5616-12.2014. Retrieved from http://www.jneurosci.org/content/34/22/7562.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Kalivas PW. Rapid, transient synaptic plasticity in addiction. Neuropharmacology. 2014;76 Pt B:276–86. doi: 10.1016/j.neuropharm.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen HW, Reissner KJ, Thomas Ca, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77(5):867–72. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith ACW, Stankeviciute NM, Hensley-simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A. 2013;110(22):9124–120. doi: 10.1073/pnas.1220591110. http://doi.org/10.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gonzalo M, Navarrete M, Perea G, Covelo A, Martin-Fernandez M, Shigemoto R, Araque A. Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cerebral Cortex. 2015;25(10):3699–3712. doi: 10.1093/cercor/bhu231. [DOI] [PubMed] [Google Scholar]
- Gordon GRJ, Baimoukhametova DV, Hewitt SA, Rajapaksha WRAKJS, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nature Neuroscience. 2005;8(8):1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Goussakov IV, Fink K, Elger CE, Beck H. Metaplasticity of Mossy Fiber Synaptic Transmission Involves Altered Release Probability. 2000;20(9):3434–3441. doi: 10.1523/JNEUROSCI.20-09-03434.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Watson JF, Cull-candy SG. Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron. 2017;94(4):713–730. doi: 10.1016/j.neuron.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Grueter Ba, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nature Neuroscience. 2010;13(12):1519–25. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundelfinger ED, Frischknecht R, Choquet D, Heine M. Converting juvenile into adult plasticity: a role for the brain's extracellular matrix. European Journal of Neuroscience. 2010;31(12):2156–2165. doi: 10.1111/j.1460-9568.2010.07253.x. [DOI] [PubMed] [Google Scholar]
- Haber M, Zhou L, Murai KK. Cooperative Astrocyte and Dendritic Spine Dynamics at Hippocampal Excitatory Synapses. Journal of Neuroscience. 2006;26(35):8881–8891. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, et al. Zhang X. Acute cannabinoids impair working memory through astroglial CB 1 receptor modulation of hippocampal LTD. Cell. 2012;148(5):1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Austelle CW, Devries W, Mithoefer O, Badran BW, George MS. What goes up, can come down: Novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Research. 2015;1628:199–209. doi: 10.1016/j.brainres.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek JA, Neuhofer D, Griffin WC, Siegel GS, Bobadilla A, Kupchik YM, Kalivas PW. Loss of Plasticity in the D2-Accumbens Pallidal Pathway Promotes Cocaine Seeking. The Journal of Neuroscience. 2017;37(4):757–767. doi: 10.1523/JNEUROSCI.2659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, Nicoll RA. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annual Review of Physiology. 2016;78(1):351–365. doi: 10.1146/annurev-physiol-021014-071753. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. The Journal of Neuroscience. 2003;23(12):4815–20. doi: 10.1523/JNEUROSCI.23-12-04815.2003. http://doi.org/23/12/4815 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbro N, Grunditz A, Wiegert JS, Oertner TG. AMPA receptors gate spine Ca2+ transients and spike-timing-dependent potentiation. Proceedings of the National Academy of Sciences. 2010;107(36):15975–15980. doi: 10.1073/pnas.1004562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH, Hsu KS. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 2011;31(11):4194–4203. doi: 10.1523/JNEUROSCI.5239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, et al. Dong Y. In Vivo Cocaine Experience Generates Silent Synapses. Neuron. 2009;63(1):40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science (New York, NY) 1992;255(5045):730–3. doi: 10.1126/science.1346729. Retrieved from http://science.sciencemag.org/content/255/5045/730.abstract. [DOI] [PubMed] [Google Scholar]
- Hunt DL, Castillo PE. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol. 2012;22(3):496–508. doi: 10.1016/j.conb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nature Reviews Neuroscience. 2012;13(11):743–757. doi: 10.1038/nrn3320.Synaptic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SS, Chung HJ. Emerging link between Alzheimer's disease and homeostatic synaptic plasticity. Neural Plasticity. 2016;2016 doi: 10.1155/2016/7969272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. Cell type-specific synaptic encoding of ethanol exposure in the nucleus accumbens shell. Neuroscience. 2014;277:184–95. doi: 10.1016/j.neuroscience.2014.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak J, Hearing M, Ingebretson A, Ebner SR, Kelly M, Fischer RA, et al. Thomas MJ. Cocaine and Amphetamine Induce Overlapping but Distinct Patterns of AMPAR Plasticity in Nucleus Accumbens Medium Spiny Neurons. Neuropsychopharmacology. 2016;41(2):464–476. doi: 10.1038/npp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones OD. Astrocyte-mediated metaplasticity in the hippocampus: Help or hindrance? Neuroscience. 2015;309:113–124. doi: 10.1016/j.neuroscience.2015.08.035. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Panlilio LV, Secci ME, Redhi GH, Charles W, Cross AJ, et al. Goldberg Steven R. The novel metabotropic glutamate receptor 2 positive allosteric modulator, AZD8529, decreases nicotine self-administration and relapse in squirrel monkeys. Biol Psychiatry. 2016;78(7):452–462. doi: 10.1016/j.biopsych.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature Reviews Neuroscience. 2009;10(8):561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The Neural Basis of Addiciton: A Pathology of Motivation and Choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Worley PF. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(14):6055–60. doi: 10.1073/pnas.0608991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The Molecular Biology of Memory Storage: A Dialogue Between Genes and Synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to Addiction Is Associated with a Persistent Impairment in Synaptic Plasticity. Science. 2010a;328(5986):1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science (New York, NY) 2010b;328(5986):1709–12. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, Manzoni OJ. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Molecular Psychiatry. 2013;18(6):729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14(9):923–930. doi: 10.1038/nm.f.1869. http://doi.org/http://www.nature.com/nm/journal/v14/n9/suppinfo/nm.f.1869_S1.html. [DOI] [PubMed] [Google Scholar]
- Katona I, Urbán GM, Wallace M, Ledent C, Jung KM, Piomelli D, et al. Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2006;26(21):5628–37. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramati M, Durand A, Girardeau P, Gutkin B, Ahmed SH. Cocaine addiction as a homeostatic reinforcement learning disorder. Psychological Review. 2017;124(2):130–153. doi: 10.1037/rev0000046. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine-seeking. Biol Psychiatry. 2010;67(1):81–84. doi: 10.1016/j.biopsych.2009.07.018.Ceftriaxone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, LaLumiere RT, Schwendt M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine-seeking. Journal of Neuroscience. 2010;30(23):7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010.Extinction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Trantham-Davidson HL, Schwendt M. The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addiction Biology. 2014;19(1):87–101. doi: 10.1111/adb.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian S, Malenka R. Simultaneous LTP of non-NMDA-and LTD of NMDA-receptor-mediated responses in the nucleus accumbens. Nature. 1994;368(6468):242–6. doi: 10.1038/368242a0. Retrieved from http://www.nature.com/nature/journal/v368/n6468/abs/368242a0.html. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine Experience Controls Bidirectional Synaptic Plasticity in the Nucleus Accumbens. Journal of Neuroscience. 2007;27(30):7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and pharmacology of striatal neurons. Annual Review of Neuroscience. 2009;32:127–47. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nature Neuroscience. 2015;18(9):1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Dingledine R. Induction and expression rules of synaptic plasticity in hippocampal interneurons. Neuropharmacology. 2011;60(5):720–729. doi: 10.1007/s11103-011-9767-z.Plastid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate Release in the Nucleus Accumbens Core Is Necessary for Heroin Seeking. Journal of Neuroscience. 2008;28(12):3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman A, Hannay T, Stratford K, Jack J. Presynaptic release probability influences the locus of long-term potentiation. Nature. 1992;360(6399):70–73. doi: 10.1038/360070a0. [DOI] [PubMed] [Google Scholar]
- Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: Silent synapse and beyond. Neuropharmacology. 2011;61(7):1060–1069. doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, et al. Dong Y. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nature Neuroscience. 2013;16(11):1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Yasuda R, Ehlers MD. Metaplasticity at Single Glutamatergic Synapses. Neuron. 2010;66(6):859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Rainnie DG. Bidirectional regulation of synaptic plasticity in the basolateral amygdala induced by the D1-like family of dopamine receptors and group II metabotropic glutamate receptors. Journal of Physiology. 2014;19:4329–4351. doi: 10.1113/jphysiol.2014.277715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proceedings of the National Academy of Sciences. 1989;86(23):9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature Reviews Neuroscience. 2002;3(3):175–90. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca 2 + -permeable AMPA receptors in synaptic plasticity and neuronal death. Trends in Neurosciences. 2007;30(3):126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330(6002):385–90. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. Dong Y. Bidirectional Modulation of Incubation of Cocaine Craving by Silent Synapse-Based Remodeling of Prefrontal Cortex to Accumbens Projections. Neuron. 2014;83(6):1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Guo CY, Yu P, Lee DYW, Han JS, Cui CL. The role of NR2B containing NMDA receptor in place preference conditioned with morphine and natural reinforcers in rats. Experimental Neurology. 2006;200(2):343–355. doi: 10.1016/j.expneurol.2006.02.117. [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type– and input-specific connectivity in the nucleus accumbens. Nature Neuroscience. 2014;17(9):1198–1207. doi: 10.1038/nn.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald JF, Jackson MF, Beazely MA. G protein-coupled receptors control NMDARs and metaplasticity in the hippocampus. Biochimica et Biophysica Acta 1768. 2007;1768:941–951. doi: 10.1016/j.bbamem.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes Erik. Neurochemistry International. 2008;52(1–2):142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mameli M, Bellone C, Brown MTC, Lüscher C. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nature Neuroscience. 2011;14(4):414–416. doi: 10.1038/nn.2763. [DOI] [PubMed] [Google Scholar]
- Mameli M, Luescher C. Synaptic plasticity and addiction: Learning mechanisms gone awry. Neuropharmacology. 2011;61(7):1052–1059. doi: 10.1016/j.neuropharm.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nature Neuroscience. 2006;9(7):868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science. 2015;349(6249):730–734. doi: 10.1126/science.aaa7945. [DOI] [PubMed] [Google Scholar]
- Marton TM, Shuler MGH, Worley PF. Homer 1a and mGluR5 phosphorylation in reward-sensitive metaplasticity : A hypothesis of neuronal selection and bidirectional synaptic plasticity. Brain Research. 2015;1628:17–28. doi: 10.1016/j.brainres.2015.06.037. [DOI] [PubMed] [Google Scholar]
- Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nature Neuroscience. 2004;7(6):585–6. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR Activation Reverses Cocaine-Induced Accumulation of Calcium-Permeable AMPA Receptors in Nucleus Accumbens Synapses via a Protein Kinase C-Dependent Mechanism. Journal of Neuroscience. 2011;31(41):14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2001;21(21):8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. http://doi.org/21/21/8655 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHail DG, Dumas TC. Multiple forms of metaplasticity at a single hippocampal synapse during late postnatal development. Developmental Cognitive Neuroscience. 2015;12:145–154. doi: 10.1016/j.dcn.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug Dependence, a Chronic Medical Illness. Journal of the American Medical Association. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, et al. Sommer WH. Rescue of Infralimbic mGluR2 Deficit Restores Control Over Drug-Seeking Behavior in Alcohol Dependence. Journal of Neuroscience. 2013;33(7):2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellentin C, Abraham WC. Priming stimulation of group II metabotropic glutamate receptors inhibits the subsequent induction of rat hippocampal long-term depression in vitro. Neuroscience Letters. 2001;307:13–16. doi: 10.1016/s0304-3940(01)01915-2. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L. Matrix Metalloproteinase-9 Controls NMDA Receptor Surface Diffusion through Integrin 1 Signaling. Journal of Neuroscience. 2009;29(18):6007–6012. doi: 10.1523/JNEUROSCI.5346-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. The Role of Glial Cells in Drug Abuse. Current Drug Abuse Review. 2009;2(1):76–82. doi: 10.2174/1874473710902010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihov Y, Hasler G. Negative Allosteric Modulators of Metabotropic Glutamate Receptors Subtype 5 in Addiction: a Therapeutic Window. International Journal of Neuropsychopharmacology. 2016;19(7):pyw002. doi: 10.1093/ijnp/pyw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/Glutamate Exchange Regulates Metabotropic Glutamate Receptor Presynaptic Inhibition of Excitatory Transmission and Vulnerability to Cocaine Seeking. Journal of Neuroscience. 2005;25(27):6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N - Acetylcysteine reverses cocaine-induced metaplasticity. Nature Neuroscience. 2009;12(2):182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen HW, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences. 2011;108(1):385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ, Kalivas PW. Signals from the Fourth Dimension Regulate Drug Relapse. Trends in Neurosciences. 2016;39(7):472–485. doi: 10.1016/j.tins.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA, Davis M. Glutamate Receptors in Extinction and Extinction-Based Therapies for Psychiatric Illness. Neuropsychopharmacology. 2011;36(1):274–293. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Huntley GW. The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learning and Memory. 2007;14(10):655–664. doi: 10.1101/lm.678307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Huntley GW. Matrix Metalloproteinase-9 Is Required for Hippocampal Late-Phase Long-Term Potentiation and Memory. Journal of Neuroscience. 2006;26(7):1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. NIDA Principles of Addiction Drug Treatment: A Research-Based Guide (Third Edition) 2012 [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends in Neurosciences. 2003;26(10):523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Neumann PA, Wang Y, Yan Y, Wang Y, Ishikawa M, Cui R, Dong Y. Cocaine-Induced Synaptic Alterations in Thalamus to Nucleus Accumbens Projection. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2016:2399–2410. doi: 10.1038/npp.2016.52. [DOI] [PMC free article] [PubMed]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Maggiore O. Transcranial direct current stimulation : State of the art 2008. Brain Stimulation. 2008;1:206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Oliet SHR, Piet R, Poulain DA. Control of Glutamate Clearance and Synaptic Efficacy by Glial Coverage of Neurons. Science. 2001;292(May):923–927. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet J, Touquet B, Pollegioni L. Glia-Derived D -Serine Controls NMDA Receptor Activity and Synaptic Memory. Cell. 2006;123(May):775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature Reviews Neuroscience. 2013;14(6):383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Park JM, Hu JH, Milshteyn A, Zhang PW, Moore CG, Park S, et al. Worley PF. A prolyl-isomerase mediates dopamine-dependent plasticity and cocaine motor sensitization. Cell. 2013;154(3):637–650. doi: 10.1016/j.cell.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O'Connor EC, Lüscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509(7501):459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Turiault M, Lüscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2012;481(7379):71–5. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- Perez-alvarez A, Navarrete M, Covelo A, Martin ED, Araque A. Structural and Functional Plasticity of Astrocyte Processes and Dendritic Spine Interactions. 2014;34(38):12738–12744. doi: 10.1523/JNEUROSCI.2401-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Otaño I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends in Neurosciences. 2005;28(5):229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Perry CJ, Reed F, Zbukvic IC, Kim JH, Lawrence AJ. The metabotropic glutamate 5 receptor is necessary for extinction of cocaine-associated cues. British Journal of Pharmacology. 2016;173(6):1085–1094. doi: 10.1111/bph.13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186(2):143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2008;28(23):6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piet R, Vargova L, Sykova E, Poulain DA, Oliet HR. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004;101(7):2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, et al. Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106(3):579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Pita-Almenar JD, Sol Collado M, Colbert CM, Eskin A. Different Mechanisms Exist for the Plasticity of Glutamate Reuptake during Early Long-Term Potentiation (LTP) and Late LTP. Journal of Neuroscience. 2006;26(41):10461–10471. doi: 10.1523/JNEUROSCI.2579-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, et al. Isaac JTR. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nature Neuroscience. 2006;9(5):602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- Poisik OV, Mannaioni G, Traynelis S, Smith Y, Conn PJ. Distinct functional roles of the metabotropic glutamate receptors 1 and 5 in the rat globus pallidus. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2003;23(1):122–30. doi: 10.1523/JNEUROSCI.23-01-00122.2003. Retrieved from http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=12514208&retmode=ref&cmd=prlinks%5Cnpapers2://publication/uuid/2F8FDA82-7D0F-4B70-9BC3-C2458E70D9DF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Srikumar BN, Mulle C. Activity-dependent synaptic plasticity of NMDA receptors. The Journal of Physiology. 2010;588(1):93–99. doi: 10.1113/jphysiol.2009.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW, Hill C. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addiction Biology. 2015;20(2):316–323. doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Jeanes ZM, Morrisett RA. Ethanol Attenuation of Long-Term Depression in the Nucleus Accumbens Can Be Overcome by Activation of TRPV1 Receptors. Alcoholism: Clinical and Experimental Research. 2014;38(11):2763–2769. doi: 10.1111/acer.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Maier EY, Buske TR, Morrisett RA. Selective alterations of NMDAR function and plasticity in D1 and D2 medium spiny neurons in the nucleus accumbens shell following chronic intermittent ethanol exposure. Neuropharmacology. 2017;112:164–171. doi: 10.1016/j.neuropharm.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G, Maroun M. Stress and Amygdala Suppression of Metaplasticity in the Medial Prefrontal Cortex. Cerebral Cortex. 2010;20(10):2433–2441. doi: 10.1093/cercor/bhp311. [DOI] [PubMed] [Google Scholar]
- Robbe D, Bockaert J, Manzoni OJ. Metabotropic glutamate receptor 2/3-dependent long-term depression in the nucleus accumbens is blocked in morphine withdrawn mice. European Journal of Neuroscience. 2002;16(11):2231–2235. doi: 10.1046/j.1460-9568.2002.02273.x. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99(12):8384–8388. doi: 10.1073/pnas.122149199. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12060781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Wolfe DJ, Kalivas PW. Glutamate Transporter GLT-1 as a Therapeutic Target for Substance Use Disorders. CNS & Neurological Disorders Drug Targets. 2015;14(6):745–56. doi: 10.1016/bs.mcb.2015.01.016.Observing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(8s2):91–117. doi: 10.1046/j.1360-0443.95.8s2.19.x. [DOI] [PubMed] [Google Scholar]
- Roloff AM, Anderson GR, Martemyanov Ka, Thayer Sa. Homer 1a gates the induction mechanism for endocannabinoid-mediated synaptic plasticity. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2010;30(8):3072–81. doi: 10.1523/JNEUROSCI.4603-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids Control the Induction of Cerebellar LTD. Neuron. 2005;48(11):647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-Serine, and endogenous synaptic modulator : Localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92(April):3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biological Psychiatry. 2015;34(5):352–359. doi: 10.1177/0963721414541462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self-Control Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith ACW, et al. Kalivas PW. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacological Reviews. 2016;68(3):816–871. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Li H, Siemsen B, Healey KL, Tran PK, Woronoff N, et al. Reissner KJ. Cocaine self-administration and extinction leads to reduced GFAP expression and morphometric features of astrocytes in the nucleus accumbens core. Biological Psychiatry. 2016;80(3):207–215. doi: 10.1016/j.biopsych.2015.12.022.Cocaine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(48):19407–12. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Scofield MD, Boger H, Hensley M, Kalivas PW. Synaptic Glutamate Spillover Due to Impaired Glutamate Uptake Mediates Heroin Relapse. Journal of Neuroscience. 2014;34(16):5649–5657. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Schachtman TR, Christoffersen GRJ. Metabotropic glutamate receptor subtype 5 antagonism in learning and memory. European Journal of Pharmacology. 2010;639(1–3):17–25. doi: 10.1016/j.ejphar.2009.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ACW, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, Kalivas PW. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nature Neuroscience. 2014;17(12):1655–1657. doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ACW, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, et al. Kalivas PW. Accumbens nNOS Interneurons Regulate Cocaine Relapse. The Journal of Neuroscience. 2017;37(4):742–756. doi: 10.1523/JNEUROSCI.2673-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ACW, Scofield MD, Kalivas PW. The tetrapartite synapse : Extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Research. 2015;1628:29–39. doi: 10.1016/j.brainres.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Current Opinion in Neurobiology. 2013;23(4):546–552. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Kalivas PW. Glutamate transport: A new bench to bedside mechanism for treating drug abuse. International Journal of Neuropsychopharmacology. 2017;20(10):797–812. doi: 10.1093/ijnp/pyx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Talani G, Mulas G, Licheri V, Fois GR, Muggironi G, et al. Diana M. Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats. Proceedings of the National Academy of Sciences. 2014;111(35):E3745–E3754. doi: 10.1073/pnas.1406768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK. LTD, LTP, and the sliding threshold for long-term synaptic plasticity. Hippocampus. 1996;6(1):35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Frontiers in Behavioral Neuroscience. 2013;7(December):1–6. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Structure and Function. 2016;221(3):1681–1689. doi: 10.1007/s00429-015-0997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniuk M, Beroun A, Lebitko T, Markina O, Leski S, Meyza K, et al. Kaczmarek L. Matrix Metalloproteinase-9 and Synaptic Plasticity in the Central Amygdala in Control of Alcohol-Seeking Behavior. Biological Psychiatry. 2017;81(11):907–917. doi: 10.1016/j.biopsych.2016.12.026. [DOI] [PubMed] [Google Scholar]
- Suska A, Lee BR, Huang YH, Dong Y, Schluter OM. Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proceedings of the National Academy of Sciences. 2013;110(2):713–718. doi: 10.1073/pnas.1206287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N, Izumi SI. Combinations of stroke neurorehabilitation to facilitate motor recovery: perspectives on Hebbian plasticity and homeostatic metaplasticity. Frontiers in Human Neuroscience. 2015;9(June) doi: 10.3389/fnhum.2015.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nature Neuroscience. 2001;4(12):1217–23. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Tigaret CM, Olivo V, Sadowski JHLP, Ashby MC, Mellor JR. Coordinated activation of distinct Ca2+ sources and metabotropic glutamate receptors encodes Hebbian synaptic plasticity. Nature Communications. 2016;7:10289. doi: 10.1038/ncomms10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomek SE, LaCrosse AL, Nemirovsky NE, Olive MF. NMDA Receptor Modulators in the Treatment of Drug Addiction. Pharmaceuticals. 2013;6:251–268. doi: 10.3390/ph6020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction Biology. 2007;12(3–4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Underlying Cause of Death 1999-2015. Centers of Disease Control and Prevention National Center for Health Statistics. 2016 Retrieved from http://wonder.cdc.gov/ucd-icd10.html on Oct, 16, 2017 7:00:11 PM.
- van Dam EJM, Kamal A, Artola A, de Graan PNE, Gispen WH, Ramakers GMJ. Group I metabotropic glutamate receptors regulate the frequency-response function of hippocampal CA1 synapses for the induction of LTP and LTD. European Journal of Neuroscience. 2004;19(1):112–118. doi: 10.1111/j.1460-9568.2004.03103.x. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M. Astroglial cradle in the life of the synapse. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1654):20130595–20130595. doi: 10.1098/rstb.2013.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Xb, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proceedings of the National Academy of Sciences. 2008;105(49):19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Side effects of repetitive transcranial magnetic stimulation. Depression and Anxiety. 2000;12(3):124–129. doi: 10.1002/1520-6394(2000)12:3<124::AID-DA3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of Group II Metabotropic Glutamate Receptor Signaling by Chronic Cocaine. Journal of Pharmacology and Experimental Therapeutics. 2002;303(2):608–615. doi: 10.1124/jpet.102.039735. Retrieved from http://jpet.aspetjournals.org/content/303/2/608.abstract. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Zhang HY, Bi GH, He Y, Gao JT, Xi ZX. Deletion of Type 2 Metabotropic Glutamate Receptor Decreases Sensitivity to Cocaine Reward in Rats. Cell Reports. 2017;20(2):319–332. doi: 10.1016/j.celrep.2017.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Calakos N. Presynaptic long-term plasticity. Frontiers in Synaptic Neuroscience. 2013;5(10):1–22. doi: 10.3389/fnsyn.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55(7):1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Yan Y, Li K, Wang Y, Huang YH, Urban NN, Dong Y. Nucleus accumbens feedforward inhibition circuit promotes cocaine self-administration. Proceedings of the National Academy of Sciences of the United States of America, Early edit. 2017 doi: 10.1073/pnas.1707822114. [DOI] [PMC free article] [PubMed]