Table 2.
Kinetic Resolutions with the CoIII-4a-OAc Catalyst
| Epoxide | Loading (mol%) |
Cond.[c] | Isolated epoxide (% yield, % ee, E-value) |
Isolated diol (% yield, % ee, E-value) |
|---|---|---|---|---|
|
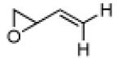
1 | A | 70, 33 S, 11 | 26, 72 R,[c] 8 |
|
|
1 | A | 44, 92 S, 20 | 56, 72 R,e19 |
|
|
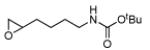
4 | B | 59, 55 (S), 16 | 39, 80 (R),[d] 15 |
|
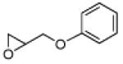
2 | B | 59, 53 (S), 13 | 37, 75 (R),[d] 11 |
|
2 | B | 45, 93 R,[e] 25 | 55, 72 S,[e],[f] 17 |
|
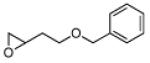
1 | B | 82, 17 S, 9 | 18, 80 R,[f] 11 |
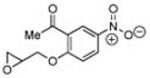
|
10 | B | 62, 28 R,[g] 3.5 | 37, 46 S,[e] 3.5 |
as for Table 1;
Assigned by comparison of optical rot'n with that of the known R-diol (ref 25);
– see notes
, Table 1;
The sign of the optical rotation was found to differ with that reported [the S-epoxide is said to levorotatory (ref 24) but the R-epoxide was found to be levorotatory here]. To confirm the absolute stereochemistry, the R-epoxide was independently from S-glycidol.
note
, Table 1.
