Abstract
Floral traits, including those invisible to humans but visible to pollinators, that increase pollination efficiency may be selected by pollinators in plant species with pollen limitation of seed production, but the importance of pollinators as selective agents on different floral traits needs to be further quantified experimentally. In the present study, we examined selective strength on flower diameter, flower height, UV bulls-eye size, sepal size and UV proportion via female fitness in Caltha scaposa, based on open-pollinated and hand-pollinated flowers, through which pollinator-mediated selection was calculated for each of floral traits. Our results suggest that seed production of C. scaposa is pollen limited in natural conditions. There was directional selection (Δβpollinator = −0.12) for larger flowers in open-pollinated flowers, while no significant selection was found in flower height, UV bulls-eye size, sepal size or UV proportion. Statistically significant selection was found in UV bulls-eye size, sepal size and UV proportion in hand-pollinated flowers, but interactions with pollinators contributed only to flower diameter. We conclude that in C. scaposa, floral traits that are subjected to selection might be driven by multiple selective agents, and suggest the importance of investigating floral traits that are invisible to human but visible to pollinators in measuring pollinator-mediated selection via male fitness.
Keywords: Pollinator-mediated selection, Floral display, Ultraviolet bulls-eye, Female fitness, Pollen limitation
The amazing floral diversity in angiosperm is generally considered to be shaped by plant–pollinator interactions (Fenster et al., 2004), and thus rapid speciation and diversification of some plant systems seem to be driven by pollinator specialization (Kay and Sargent, 2009). For example, the nectar spur in Aquilegia, defined as a key innovation allowing greater pollinator specialization (Hodges, 1997), leads to the speciation and diversification (Whittall and Hodges, 2007), which could be then reinforced by pollinator-mediated reproductive isolation (Kay and Sargent, 2009, Weber and Strauss, 2016). Theoretically, pollinator-mediated natural selection favors floral traits that maximize reproductive fitness via pollen export and import (Morgan, 1992), and thus pollinator-mediated selection on floral traits should be strong if reproductive success is limited (Ashman et al., 2004). Accordingly, the wide occurrence of pollen limitation of seed production in plants (Larson and Barrett, 2000, Knight et al., 2005) suggests that pollinators might drive macro-evolution of floral traits (Sapir and Armbruster, 2010). Thus, understanding which floral traits are under natural selection would fill a gap in our knowledge of whether or not pollinators are the agents of natural selection within populations.
A number of floral traits are found to be selected by pollinators, constituting a major component in the definition of pollination syndrome, in which flower color is paid more attention based on the broad association between flower color and pollinator functional group (Fenster et al., 2004). For example, birds generally but not always prefer red flowers, and bees generally prefer yellow, green and blue flowers (Zhang, 2003). However, it should be noted that classification of flower color is based on human eyes, while that color might be quite different to a pollinator's eyes. For example, flowers of most species in Potentilla are yellow, but some of them show quite different UV absorption patterns (Naruhashi and Ikeda, 1999, Koski and Ashman, 2016). Despite the fact that this kind of UV absorbing floral type is generally correlated with bioclimatic factors (Koski and Ashman, 2015b, Koski and Ashman, 2016), it has been suggested that flowers with larger UV absorption patterns may be perceived (Chittka et al., 1994, Briscoe and Chittka, 2001, Kevan et al., 2001, Koski and Ashman, 2014, Sheehan et al., 2016) and preferred (Horth et al., 2014, Brock et al., 2016) by insects as the bulls-eye patterns are thought to act as nectar guides. Accordingly, we cannot judge pollination syndrome of a flower color based on human eyes only, and measurements of phenotypic selection on floral traits should include these “cryptic” floral traits, like the UV bulls-eye.
Caltha is a small but widely distributed genus in Ranunculaceae family (Wang et al., 2001), and flowers of Catha palustris are visited by a wide spectrum of insects in Canada, including bees, beetles, flies, ants, thrip, bug, butterfly and sawfly (Judd, 1964), indicating a generalized pollination syndrome in this genus as floral traits among different species of Caltha show great similarity. Although the sepal of Caltha scaposa is consistently yellow under visible light (Fig. 1a), when we photographed flowers of C. scaposa using a modified camera equipped with a lens allowing UV only in the field, we found that the sepal base absorbed UV spectrum (Fig. 1b) but the sepal apex reflected UV spectrum (Fig. 1b), an indicator of a UV bulls-eye pattern. Furthermore, our preliminary observations suggested that there were great variations in bulls-eye size within one population. Therefore, in the present study, we performed field experiments to measure phenotypic selection on floral traits via female fitness, with emphasis on examining whether or not UV bulls-eye is the target of natural selection in C. scaposa.
Fig. 1.
One flower of Caltha scaposa in visible light (a) and UV light (b), and one dried sepal in visible light (c) and UV light (d). The black parts of sepals in UV light show the UV absorbing pattern.
1. Material and methods
1.1. Plant species
C. scaposa J. D. Hooker & Thomson is a perennial herb in the buttercup family (Ranunculaceae), inhabiting alpine wet meadows with altitudes ranging from 2800 m to 4100 m (Wang et al., 2001). Flower of C. scaposa is usually solitary and terminal, and the petal-like sepal is yellow, which might act as the role of petal in this species. C. scaposa begins to flower in June, and our research was carried out from June to July, 2016. The study population (26°37′42″N, 99°43′15″E) is located at Laojunshan, Lijiang, Yunnan province, with an altitude of ca. 4000 m. Our preliminary observations suggest that C. scaposa is characterized by protandry and herkogamy, indicating that pollinators are necessary for seed production because a combination of dichogamy and herkogamy can prevent autonomous selfing successfully (Duan et al., 2005, Guo et al., 2014). Further observations suggest that flowers of C. scaposa may be visited by bumblebees, honeybees, solitary bees and flies, indicating a generalized pollination syndrome for this species.
1.2. Field experiment and measurements
Because plants with more than one flower were rare in the study population, we only chose plants with one flower. In mid-June 2016, a total of 150 plants with buds were selected and tagged. Fifty plants were randomly assigned for supplemental hand-pollination with pollen grain from plants that were at least 20 m from the receptive plants to ensure outcrossing, and 100 plants were assigned to open pollination as a control. The selected plants were visited every day, and those plants subjected to hand pollination were pollinated by rubbing anthers across the stigmas until all stigmas within one flower received supplemental pollen at least once.
When each flower opened completely, we measured the flower diameter using a caliper, and flower height (distance from the ground to the flower) using a ruler. When flowers began wilting, we collected one sepal from each plant and kept it in a book to make it flat and dried because UV bulls-eye floral guides could also be photographed on dried flowers (Koski and Ashman, 2016). In the laboratory, each sepal was put on a white-colored paper and photographed twice with a modified camera. First, the sepal was photographed under visible light, and then under additional UV spectrum with a lens allowing UV only to record the UV bulls-eye. Each picture was then treated, using Adobe Photoshop CS2, by removing the background of white paper, which allowed the color channels (red, blue and green) to be obtained in ImageJ (Rasband, 2012). Using the red channel by setting different thresholds, we measured the UV-absorbing area and sepal area, and then UV proportion was calculated as the (UV-absorbing area/sepal area).
1.3. Statistical analysis
The effects of pollination treatment on plant performance (including seed number, flower diameter, flower height, UV bulls-eye size, sepal size and UV proportion) were examined using independent sample t test.
Multiple regression analyses were employed to estimate phenotypic selection on floral traits via female fitness (Lande and Arnold, 1983). For each treatment, female fitness was transformed to relative female fitness by dividing the mean seed production with individual seed production, and flower traits were standardized with a mean of 0 and a standard deviation of 1. Initially, quadratic terms (γ) were also included to measure nonlinear selection, but none of the quadratic gradients was statistically significant. Therefore, we only report linear gradients here.
ANCOVA was also used to examine whether or not pollination treatment changed selection gradient, with relative female fitness as the dependent variable. In this model, the standardized floral traits (flower diameter, flower height, UV bulls-eye size, sepal size and UV proportion), pollination treatment (control and hand pollination) and floral trait × pollination treatment interactions were treated as independent variables. To quantify the gradients of pollinator-mediated selection for each traits, the estimated selection gradient for plants receiving supplemental hand pollination (βHP) was subtracted from that for open-pollinated controls (βC), Δβpoll = βC − βHP.
2. Results
Because animals destroyed some labeled plants, the sample size of open-pollinated flowers and hand-pollinated flowers was reduced to 75 and 21, respectively. Hand-pollination increased seed number significantly in comparison with open pollination (Table 1), indicating pollen limitation of seed production in C. scaposa. No significant difference was found in each of five floral traits (flower diameter, flower height, UV bulls-eye size, sepal size and UV proportion) between open-pollinated flowers and hand-pollinated flowers (Table 1).
Table 1.
Comparisons of traits (mean ± SE) between open-pollinated flowers (control) and hand-pollinated flowers of Caltha scaposa based on independent sample t-test.
| Treatment | Hand pollination (21) | Control (75) | P |
|---|---|---|---|
| Seed production | 91.00 ± 8.32 | 48.37 ± 3.10 | <0.01 |
| Flower diameter (mm) | 27.06 ± 0.83 | 28.33 ± 0.30 | 0.16 |
| Flower height (cm) | 14.86 ± 0.55 | 14.81 ± 0.38 | 0.96 |
| UV bulls-eye size | 15.58 ± 0.68 | 16.68 ± 0.46 | 0.24 |
| Sepal size | 33.40 ± 1.46 | 34.23 ± 0.63 | 0.55 |
| UV proportion | 0.47 ± 0.02 | 0.48 ± 0.01 | 0.43 |
Generally, floral traits were positively correlated, and a significant correlation was found between UV bulls-eye size and floral traits (Table 2). Specifically, UV bulls-eye size was significantly correlated with flower diameter and sepal size (Table 2), indicating that UV bulls-eye tended to be large in large flowers. In addition, UV proportion was positively correlated with UV flower diameter and bulls-eye size (Table 2).
Table 2.
Correlation analysis among floral traits of Caltha scaposa based on all sampled plants. Asterisk indicates the significant correlation at the 0.05 level.
| Flower diameter | Flower height | UV bulls-eye size | Sepal size | UV proportion | |
|---|---|---|---|---|---|
| Flower diameter | – | 0.171 | 0.209* | 0.067 | 0.211* |
| Flower height | – | – | 0.020 | 0.087 | −0.042 |
| UV bulls-eye size | – | – | – | 0.605* | 0.661* |
| Sepal size | – | – | – | – | −0.167 |
| UV proportion | – | – | – | – | – |
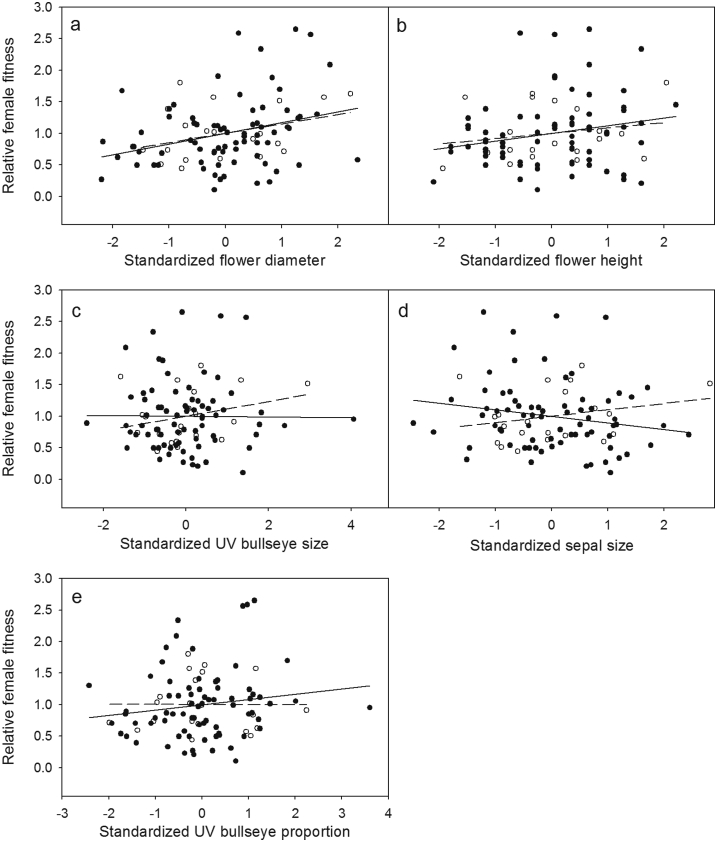
Under natural conditions, we found directional selection on one of five floral traits. Plants with larger flower diameter showed higher relative female fitness (Fig. 2a). There was no significant selection found for flower height (Fig. 2b), UV bulls-eye size (Fig. 2c), sepal size (Fig. 2d) and UV proportion (Fig. 2e) (open-pollinated flowers, Table 3). Despite statistically significant selection on UV bulls-eye size, sepal size and UV proportion in hand-pollinated flowers, only interactions with pollinators contributed to flower diameter (Table 3).
Fig. 2.
Linear phenotypic selection on standardized flower diameter (a), flower height (b), UV bulls-eye size (c), sepal size (d) and UV proportion (e) in open-pollinated (dots, solid lines) and hand-pollinated (open circle, dashed lines) flowers via relative female fitness in Caltha scaposa.
Table 3.
Linear selection gradients (±SE) for open-pollinated flowers (βC) and hand-pollinated flowers (βHP) in Caltha scaposa. Δβpollinator suggests the strength of pollinator-mediated selection (Δβpollinator = βC − βHP), and trait × pollination interactions based on ANCOVA are indicated. Asterisk indicates the significant difference at the 0.05 level.
| Trait | βC (n = 76) | βHP (n = 21) | Δβpollinator | PTrait×pollination |
|---|---|---|---|---|
| Flower diameter | 0.27 ± 0.063* | 0.39 ± 0.082 | −0.12 | 0.02 |
| Flower height | 0.18 ± 0.061 | 0.37 ± 0.089 | −0.19 | 0.13 |
| UV bulls-eye size | −0.08 ± 0.061 | 6.05 ± 0.877* | −6.13 | 0.130 |
| Sepal size | −0.16 ± 0.062 | −5.97 ± 0.899* | 5.79 | 0.14 |
| UV proportion | 0.14 ± 0.063 | −4.63 ± 0.678* | 4.77 | 0.13 |
3. Discussion
Animal-pollinated plant species that experience pollen limitation in seed production might enhance allocation to attract pollinators, thus resulting in pollinator-mediated selection on floral traits (Ashman and Morgan, 2004). In our study, we found that hand pollination increased seed production significantly compared with open-pollinated flowers, indicating that seed production in Caltha scarposa is pollen-limited. Thus, natural selection on floral traits that increase attraction to pollinators was expected in C. scaposa. Large floral display size has been shown to be attractive to pollinators from a long distance, and directional natural selection on larger floral display size has been documented previously (Sandring and Ågren, 2009, Parachnowitsch and Kessler, 2010, Sletvold et al., 2010). In C. scaposa, there was generally one flower on one plant, so we did not consider pollinator-mediated selection on number of flowers in our study. Aside from number of flowers, flower size may also contribute to floral display size. In line with this expectation, we found that open-pollinated flowers resulted in an increase flower diameter in C. scaposa, indicating that directional selection acts on floral display size. However, when pollinator-mediated selection was considered only, we found negative selection on flower diameter (Δβpollinator = −0.12), which might indicate that there were other selective agents driving the evolution of flower diameter in C. scaposa. For example, herbivores have been thought to drive conflicting selection with pollinators by altering pollinator behaviors, as has been shown when floral traits attractive to pollinators also attract herbivores (Gomez, 2003, Irwin, 2006, Sandring et al., 2007). In addition, some floral traits might be selected exclusively by herbivores instead of pollinators (Parachnowitsch and Caruso, 2008). Accordingly, the negative selection on flower diameter mediated by pollinator in the present study could indicate that selective strength on flower diameter of other selective agents might exceed that of pollinators in C. scaposa, although this needs to be demonstrated in future studies.
Excluding floral size, plant height might also contribute to floral display size to a certain degree as reproductive success of plant species can be affected by vegetation height (Toräng et al., 2006), and taller plants would make flowers more attractive to pollinators from a long distance. As a result, natural selection may favor taller plants (O'Connell and Johnston, 1998). For example, in the deceptive orchid Dactylorhiza lapponica, pollinator-mediated selection accounted for 76% of the observed selection on plant height (Sletvold et al., 2010). However, in the present study on C. scaposa, we did not find natural selection on flower height via female fitness, which could be attributed to the habitats and the flowering phenology of this species. At our study site, C. scaposa occupies alpine meadow where the vegetation height generally does not exceed plant height of our study species. Furthermore, C. scaposa is an early-flowering species in the alpine community, and yellow flowers might be more obvious in the green background of alpine meadow. Collectively, although plant height is considered to be driven by natural selection, this expectation might be environment- and species-dependent and not applicable to C. scaposa.
Although UV floral patterns on petals are invisible to human eyes, they are visible to insect pollinators and considered to be a stable and heritable trait in plant species (Yoshioka et al., 2005, Syafaruddin et al., 2006, Koski and Ashman, 2013). In fact, UV floral patterns have been suggested to be treated as an important character for plant taxonomy because of their stability in herbarium specimens (Eisner et al., 1973). UV patterns on petals can be perceived by bees (Chittka, 1992), birds (Bennett and Cuthill, 1994), moths (Sheehan et al., 2016) and flies (Koski and Ashman, 2015a). Furthermore, both bees and flies have been shown to prefer flowers with large UV bulls-eye patterns (Horth et al., 2014, Koski and Ashman, 2015a, Brock et al., 2016). Therefore, we predicted that natural selection would drive the evolution of flowers with larger bulls-eye patterns in C. scaposa. However, in the present study, we did not find any phenotypic selection on floral traits related to UV bulls-eye size in open-pollinated flowers, and also did not find any selective strength mediated by pollinators (Table 3). These results might suggest that changes of UV bulls-eye size could be subject to selection via male fitness instead of female fitness (Koski and Ashman, 2015a). In previous studies, flowers with larger UV bulls-eye patterns received pollinator visits two times more than those with small UV bulls-eye patterns, although the pollinator visitation rate was high for flowers with small UV bulls-eye patterns (Horth et al., 2014, Brock et al., 2016). In light of pollen presentation theory, when pollinators are frequent, plants achieve higher male fitness by presenting pollen grains to more pollinators (Thomson et al., 2000, Castellanos et al., 2006). Therefore, evolution of UV bulls-eye size might be driven by natural selection via male fitness in C. scaposa, but this needs further research.
Collectively, in the present study, we examined natural selection on five floral traits (flower diameter, flower height, UV bulls-eye size, sepal size and UV proportion) of C. scaposa via female fitness by combining field experiments and UV photography. We only found significant pollinator-mediated selection on floral diameter. We did not find any significant natural selection on UV-related floral traits (UV bulls-eye size or UV proportion) via female fitness, even though UV floral pattern and size are considered to be selected by pollinators. Our study emphasizes the importance of investigating floral traits that are invisible to human but visible to pollinators in measuring pollinator-mediated selection on floral traits, and suggests the necessity of examining male fitness in surveying natural selection on floral traits.
Acknowledgments
We are grateful to Mr. Ying-Hong Yang for his help in field work. This work was financially supported by National Natural Science Foundation of China (Grant numbers: 41271058, 31460096, 31570385).
(Editor: David Boufford)
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
Contributor Information
Yongping Yang, Email: yangyp@mail.kib.ac.cn.
Yuanwen Duan, Email: duanyw@mail.kib.ac.cn.
References
- Ashman T.-L., Knight T.M., Steets J.A. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Ashman T.-L., Morgan M.T. Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context? Proc. R. Soc. Lond. B Biol. Sci. 2004;271:553–559. doi: 10.1098/rspb.2003.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A.T.D., Cuthill I.C. Ultraviolet vision in birds: what is its function? Vis. Res. 1994;34:1471–1478. doi: 10.1016/0042-6989(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Briscoe A.D., Chittka L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. [DOI] [PubMed] [Google Scholar]
- Brock M.T., Lucas L.K., Anderson N.A. Genetic architecture, biochemical underpinnings and ecological impact of floral UV patterning. Mol. Ecol. 2016;25:1122–1140. doi: 10.1111/mec.13542. [DOI] [PubMed] [Google Scholar]
- Castellanos M.C., Wilson P., Keller S.J. Anther evolution: pollen presentation strategies when pollinators differ. Am. Nat. 2006;167:288–296. doi: 10.1086/498854. [DOI] [PubMed] [Google Scholar]
- Chittka L. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. J. Comp. Physiol. A. 1992;170:533–543. [Google Scholar]
- Chittka L., Shmida A., Troje N. Ultraviolet as a component of flower reflections, and the colour perception of hymenoptera. Vis. Res. 1994;34:1489–1508. doi: 10.1016/0042-6989(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Duan Y.-W., He Y.-P., Liu J.-Q. Reproductive ecology of the Qinghai-Tibet Plateau endemic Gentiana straminea (Gentianaceae), a hermaphrodite perennial characterized by herkogamy and dichogamy. Acta Oecol. 2005;27:225–232. [Google Scholar]
- Eisner T., Eisner M., Hyypio P.A. Ultraviolet patterns of flowers visible as fluorescent patterns in pressed herbarium specimens. Science. 1973;179:486–487. doi: 10.1126/science.179.4072.486. [DOI] [PubMed] [Google Scholar]
- Fenster C.B., Armbruster W.S., Wilson P. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 2004;35:375–403. [Google Scholar]
- Gomez J.M. Herbivory reduces the strength of pollinator-mediated selection in the mediterranean herb Erysimum mediobispanicum: consequences for plant specialization. Am. Nat. 2003;162:242–256. doi: 10.1086/376574. [DOI] [PubMed] [Google Scholar]
- Guo W., Wang L.-L., Sun S. Sexual interference in two Chamerion species with contrasting modes of movement herkogamy. J. Syst. Evol. 2014;52:355–362. [Google Scholar]
- Hodges S.A. Floral nectar spurs and diversification. Int. J. Plant Sci. 1997;158:S81–S88. [Google Scholar]
- Horth L., Campbell L., Bray R. Wild bees preferentially visit Rudbeckia flower heads with exaggerated ultraviolet absorbing floral guides. Biol. Open. 2014;3:221–230. doi: 10.1242/bio.20146445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin R.E. The consequences of direct versus indirect species interactions to selection on traits: pollination and nectar robbing in Ipomopsis aggregata. Am. Nat. 2006;167:315–328. doi: 10.1086/499377. [DOI] [PubMed] [Google Scholar]
- Judd W.W. Insects associated with flowering marsh marigold, Caltha palustris L., at London, Ontario. Can. Entomol. 1964;96:1472–1476. [Google Scholar]
- Kay K.M., Sargent R.D. The role of animal pollination in plant speciation: integrating ecology, geography, and genetics. Annu. Rev. Ecol. Evol. Syst. 2009;40:637–656. [Google Scholar]
- Kevan P.G., Chittka L., Dyer A.G. Limits to the salience of ultraviolet: lessons from colour vision in bees and birds. J. Exp. Biol. 2001;204:2571–2580. doi: 10.1242/jeb.204.14.2571. [DOI] [PubMed] [Google Scholar]
- Knight T.M., Steets J.A., Vamosi J.C. Pollen limitation of plant reproduction: pattern and process. Annu. Rev. Ecol. Evol. Syst. 2005;36:467–497. [Google Scholar]
- Koski M.H., Ashman T.-L. Quantitative variation, heritability, and trait correlations for ultraviolet floral traits in Argentina anserina (Rosaceae): implications for floral evolution. Int. J. Plant Sci. 2013;174:1109–1120. [Google Scholar]
- Koski M.H., Ashman T.-L. Dissecting pollinator responses to a ubiquitous ultraviolet floral pattern in the wild. Funct. Ecol. 2014;28:868–877. [Google Scholar]
- Koski M.H., Ashman T.-L. An altitudinal cline in UV floral pattern corresponds with a behavioral change of a generalist pollinator assemblage. Ecology. 2015;96:3343–3353. doi: 10.1890/15-0242.1. [DOI] [PubMed] [Google Scholar]
- Koski M.H., Ashman T.-L. Floral pigmentation patterns provide an example of Gloger's rule in plants. Nat. Plants. 2015;1:14007. doi: 10.1038/nplants.2014.7. [DOI] [PubMed] [Google Scholar]
- Koski M.H., Ashman T.-L. Macroevolutionary patterns of ultraviolet floral pigmentation explained by geography and associated bioclimatic factors. New Phytol. 2016;211:708–718. doi: 10.1111/nph.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R., Arnold S.J. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Larson B.M.H., Barrett S.C.H. A comparative analysis of pollen limitation in flowering plants. Biol. J. Linnean Soc. 2000;69:503–520. [Google Scholar]
- Morgan M.T. The evolution of traits influencing male and female fertility in outcrossing plants. Am. Nat. 1992;139:1022–1051. [Google Scholar]
- Naruhashi N., Ikeda H. Variation in nectar guides in Himalayan Potentilla (Rosaceae) Himalayan Plants. 1999;3:23–29. [Google Scholar]
- O'Connell L.M., Johnston M.O. Male and female pollination success in a deceptive orchid, a selection study. Ecology. 1998;79:1246–1260. [Google Scholar]
- Parachnowitsch A.L., Caruso C.M. Predispersal seed herbivores, not pollinators, exert selection on floral traits via female fitness. Ecology. 2008;89:1802–1810. doi: 10.1890/07-0555.1. [DOI] [PubMed] [Google Scholar]
- Parachnowitsch A.L., Kessler A. Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytol. 2010;188:393–402. doi: 10.1111/j.1469-8137.2010.03410.x. [DOI] [PubMed] [Google Scholar]
- Rasband W.S. U.S. National Institute of Health; Bethsda, MD: 2012. ImageJ.https://imagej.nih.gov/ij/ [Google Scholar]
- Sandring S., Ågren J. Pollinator-mediated selection on floral display and flowering time in the perennial herb Arabidopsis lyrata. Evolution. 2009;63:1292–1300. doi: 10.1111/j.1558-5646.2009.00624.x. [DOI] [PubMed] [Google Scholar]
- Sandring S., RiihimÄKi M.A., Savolainen O. Selection on flowering time and floral display in an alpine and a lowland population of Arabidopsis lyrata. J. Evol. Biol. 2007;20:558–567. doi: 10.1111/j.1420-9101.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- Sapir Y., Armbruster S.W. Pollinator-mediated selection and floral evolution: from pollination ecology to macroevolution. New Phytol. 2010;188:303–306. doi: 10.1111/j.1469-8137.2010.03467.x. [DOI] [PubMed] [Google Scholar]
- Sheehan H., Moser M., Klahre U. MYB-FL controls gain and loss of floral UV absorbance, a key trait affecting pollinator preference and reproductive isolation. Nat. Genet. 2016;48:159–166. doi: 10.1038/ng.3462. [DOI] [PubMed] [Google Scholar]
- Sletvold N., Grindeland J.M., Ågren J. Pollinator-mediated selection on floral display, spur length and flowering phenology in the deceptive orchid Dactylorhiza lapponica. New Phytol. 2010;188:385–392. doi: 10.1111/j.1469-8137.2010.03296.x. [DOI] [PubMed] [Google Scholar]
- Syafaruddin, Kobayashi K., Yoshioka Y. Estimation of heritability of the nectar guide of flowers in Brassica rapa L. Breed. Sci. 2006;56:75–79. [Google Scholar]
- Thomson J.D., Wilson P., Valenzuela M. Pollen presentation and pollination syndromes, with special reference to Penstemon. Plant Species Biol. 2000;15:11–29. [Google Scholar]
- Toräng P., Ehrlén J., Ågren J. Facilitation in an insect-pollinated herb with a floral display dimorphism. Ecology. 2006;87:2113–2117. doi: 10.1890/0012-9658(2006)87[2113:fiaihw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wang W.-C., Fu D.-Z., Li L.-Q. Ranunculaceae. In: Wu Z.Y., Raven P.H., editors. Flora of China. Science Press & Missouri Botanical Garden Press; Beijing & St. Louis: 2001. [Google Scholar]
- Weber M.G., Strauss S.Y. Coexistence in close relatives: beyond competition and reproductive isolation in sister taxa. Annu. Rev. Ecol. Evol. Syst. 2016;47:359–381. [Google Scholar]
- Whittall J.B., Hodges S.A. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–709. doi: 10.1038/nature05857. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y., Horisaki A., Kobayashi K. Intraspecific variation in the ultraviolet colour proportion of flowers in Brassica rapa L. Plant Breed. 2005;124:551–556. [Google Scholar]
- Zhang D.-Y. Plant reproductive ecology. In: Zhang D.-Y., editor. Plant Life-history Evolution and Reproductive Ecology. Science Press; Beijing: 2003. [Google Scholar]