Abstract
Background
Cannabinoids have shown promise for the treatment of intractable pain states and may represent an alternative pharmacotherapy for pain management. A growing body of clinical evidence suggests a role for sex in pain perception and in cannabinoid response. We examined cannabinoid sensitivity and tolerance in male and female mice expressing a desensitization-resistant form (S426A/S430A) of the cannabinoid type 1 (CB1) receptor.
Materials and Methods
Mice were assessed for acute and inflammatory nociceptive behaviors in the formalin test following pretreatment with either vehicle or mixed CB1/CB2 receptor agonists, Δ9-THC (1–6 mg/kg) or CP55,940 (0.06–0.2 mg/kg). Tolerance to the effects of 6 mg/kg Δ9-THC or 0.1 mg/kg CP55,940 was examined via the formalin test following chronic daily dosing.
Results
Female mice showed decreased sensitivity to the effects of Δ9-THC and CP55,940 compared to male controls. The S426A/S430A mutation increased the attenuation of nociceptive behaviors for both agonists in both sexes. Female mice displayed delayed tolerance to Δ9-THC compared to male mice while the S426A/S430A mutation conferred a delay in tolerance to Δ9-THC in both sexes. Male S426A/S430A mutant mice also display resistance to tolerance to CP55,940 compared to wild-type controls.
Conclusion
This study demonstrates sex and genotype differences in response for two different cannabinoid agonists. The results underscore the importance of including both male and female subjects in pre-clinical studies of pain and cannabinoid pharmacology.
Keywords: delta-9-tetrahydrocannabinol; CP55,940; inflammatory pain; formalin test; tolerance; sex differences
Introduction
Approximately 25.3 million US adults suffer from chronic pain (defined as daily pain exceeding three months duration) [1]. While opioids provide some relief for patients with acute and cancer-related pain, the effectiveness of prolonged opioid use for managing chronic, non-cancer pain is less clear [2]. Furthermore, the long-term risks of continued opioid use, including; tolerance, dependence, addiction, and overdose may outweigh their therapeutic potential [3]. Cannabinoids have shown promise in the management of a number of difficult-to-treat pain states [4–5], and may offer a non-opioid alternative for long-term management of chronic inflammatory pain.
Despite the therapeutic potential of cannabinoids, tolerance to the effects of delta-9-tetrahydrocannabinol (Δ9-THC) can occur in heavy users [6–8]. Likewise, rapid tolerance to the antinociceptive effects of Δ9-THC has been demonstrated in preclinical rodent studies [9,10]. Tolerance to Δ9-THC can occur through a combination of neuroadaptations that include desensitization and down-regulation of the cannabinoid type 1 (CB1) receptor. Desensitization-resistant S426A/S430A “knock-in” mice exhibit an increased acute response and delayed tolerance to the antinociceptive and hypothermic effects of Δ9-THC [9]. However, the effect of this mutation on sensitivity and tolerance to the antinociceptive effects of Δ9-THC in female mice or using models of pathological pain has not been examined. Given the potential therapeutic use of cannabinoids as analgesics, it is important to understand the mechanisms underlying tolerance.
Clinically, women report a higher prevalence of chronic pain [1] and greater levels of both experimentally-induced [11] and post-operative [12] pain than males. A growing body of evidence suggests a role for sex in mediating the magnitude of cannabinoid response [13–15] and cannabis abuse potential [13,16–18]. Therefore, the objective of this study was to determine whether tolerance to the antinociceptive effects of Δ9-THC, a strongly desensitizing, partial CB1R agonist or CP55,940, a strongly internalizing, full CB1R agonist were altered in wild-type or S426A/S430A mutant mice in a sex-specific manner in a mouse model of inflammatory pain.
Materials and Methods
Subjects
Subjects included 353 10–16 week old male and female age-matched S426A/S430A mutant and wild-type mice on a C57BL/6 background. S426A/S430A mutant mice were previously produced by substituting serines at residues 426 and 430 with alanines in the carboxy terminal of the CB1 receptor [9]. Mice were group-housed on a 12:12 hour light/dark cycle with ad libitum access to food and water. All animal care and experimental procedures used were approved and conducted in accordance with The Guide for the Care and Use of Laboratory Animals, 8th edition and with approval from Penn State University’s Institutional Animal Care and Use Committee (IACUC).
Drugs
5-(1,1-demethylheptyl)-2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]-phenol [(−)-CP55,940; Cayman Chemical, Ann Arbor, MI, catalog #90084] and delta-9-tetrahydrocannabinol (Δ9-THC) (National Institute on Drug Abuse Drug Supply, Bethesda, MD) were dissolved in 0.9% physiological saline, 5% Cremaphor EL, and 5% ethanol (18:1:1 v/v/v) and administered intraperitoneally (IP) in an injection volume of 10 ml/kg.
Formalin Test
Male and female S426A/S430A and wild-type littermate mice were assessed for differences in nociceptive behaviors using the formalin test. Mice were injected IP with either vehicle, Δ9-THC or CP55,940 sixty minutes prior to intraplantar injection of 10 µl of 2.5% formalin. Mice were acclimated to the testing chamber for 15 minutes prior to formalin injection and were returned to the chamber immediately following formalin injection and video recorded using a high-definition digital camera (Logitech, Newark, CA). Nociceptive behaviors were quantified within 12 consecutive 5-minute bins based as previously described [19]. The area under the curve (AUC) was calculated for the acute phase (0–15 min; phase I) and the inflammatory phase (15–60 min; phase II).
Experimental Procedures
Dose response curves were generated by dosing naïve mice with Δ9-THC (1, 3, or 6 mg/kg), CP55,940 (0.06, 0.1, or 0.2 mg/kg) or vehicle 60 minutes prior to formalin testing. Tolerance was assessed by repeatedly injecting mice once daily with 6 mg/kg Δ9-THC for 3, 7, 10 or 14 days or 0.1 mg/kg CP55,940 for 10, 14, 21 or 28 days. On the day of formalin testing, mice were administered the final dose of Δ9-THC or CP55,940 60 minutes prior to the formalin test.
Statistical Analysis
Data were analyzed with GraphPad Prism 7 (GraphPad Software, Inc. La Jolla, CA) using a two-way analysis of variance (ANOVA) with genotype and day/dose/sex as the main factors. Mice in the vehicle group with an inflammatory phase AUC >2.5 standard deviations above or >1.5 standard deviations below the mean were excluded from analysis. In addition, a single female S426A/430A mouse treated with 0.2 mg/kg CP55,940 was determined to be an outlier using the modified Thompson Tau test and was also excluded. Dunnett’s post hoc were used for day and dose by genotype ANOVAs and Tukey’s post hoc for sex by genotype comparisons within each dose. Data were expressed as mean +/− standard error of the mean (SEM). For all tests, statistical significance was set at p<0.05.
Results
No sex or genotype differences in baseline nociceptive behaviors were found as a function of sex (p=0.699) or genotype (p=0.937) following vehicle administration.
Dose response to Δ9-THC
Phase I (Acute phase)
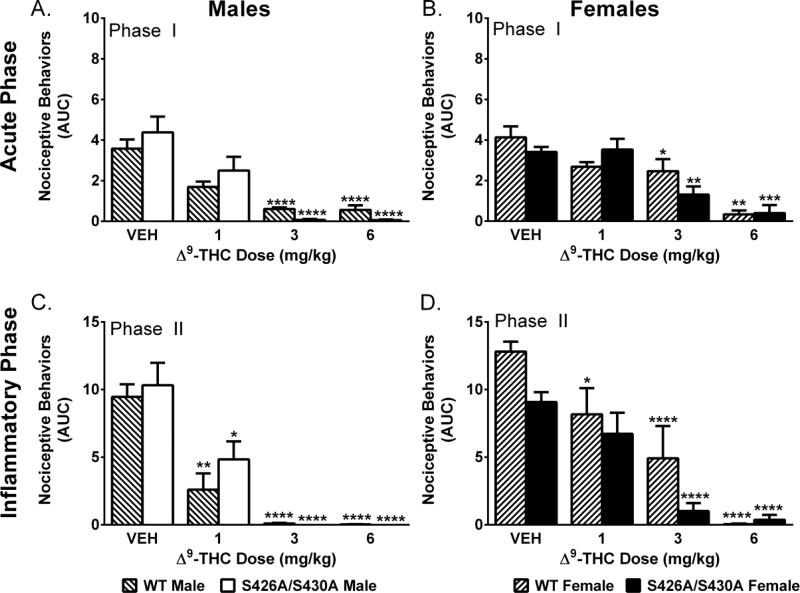
Two-way ANOVA revealed main effects of Δ9-THC dose for male (F(3,48)=12.64; p<0.001) and female (F(3,43)=21.74; p<0.001) mice (Fig 1A–B). Separate two-way ANOVAs at doses of 1, 3 and 6 mg/kg Δ9-THC revealed that females were less sensitive to the effects of 1 mg/kg (F(1,16)=4.78; p=0.044) and 3 mg/kg (F(1,16)=18.32; p<0.001) Δ9-THC. S426A/S430A mutant mice showed an enhanced response to 3 mg/kg Δ9-THC (F(1,16)=5.44; p=0.033).
Figure 1. Δ9-THC in the formalin test.
Effects of Δ9-THC (n=4–5 mice/dose/group) on the acute (Phase I; A–B) and inflammatory (Phase II; C–D) phase of the formalin test in male (A,C) and female (B,D) mice compared to vehicle (VEH; n=9–15) in wild-type (WT) (lined bars) and S426A/S430A mutant (solid bars) mice. Bars represent mean +/− SEM. Asterisk(s) indicate significant differences from vehicle; * p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Phase II (Inflammatory phase)
We found main effects of Δ9-THC dose for both male (F(3,48)=19.35; p<0.001) and female (F(3,43)=29.31; p<0.001) mice. Female mice displayed a main effect of genotype (F(1,43)=6.23; p=0.0165) with S426A/S430A female mutants showing an increased response to Δ9-THC compared to wild-type controls. Separate two-way ANOVAs at each Δ9-THC dose (1, 3, and 6 mg/kg) found that females were less sensitive to the effects of 1 mg/kg (F(1,16)=5.93; p=0.027) and 3 mg/kg (F(1,16)=5.69; p=0.030) Δ9-THC compared to males (Fig 1C–D).
Dose Response to CP55,940
Phase I (Acute phase)
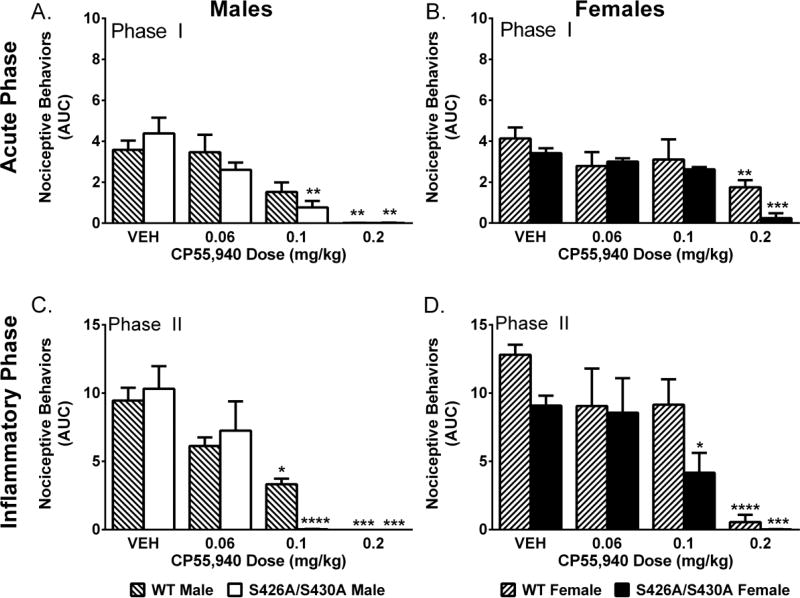
Two-way ANOVA revealed main effects of CP55,940 dose for male (F(3,42)=9.04; p<0.001) and female (F(3,42)=9.76; p<0.001) mice. Separate two-way ANOVAs found that female mice were less sensitive to the effects of 0.1 mg/kg (F(1,15)=15.34; p=0.001) and 0.2 mg/kg (F(1,10)=11.48; p=0.007) CP55,940. S426A/S430A mutant mice exhibit an enhanced response to 0.1 mg/kg (F(1,14)=10.57; p=0.005) and 0.2 mg/kg (F(1,10)=6.69; p=0.027) CP55,940 compared to wild-type controls. A sex-by-genotype interaction (F(1,10)=6.75; p=0.027) at 0.2 mg/kg CP55,940 followed by post hoc testing revealed that female wild-type mice were less sensitive to the effects of 0.2 mg/kg compared to male wild-type (p=0.005), male mutant (p=0.005) and female mutant (p=0.013) mice (Fig 2A–B).
Figure 2. CP55,940 produced a dose-dependent attenuation of nociceptive behavior in the formalin test.
Effects of CP55,940 (n=3–5 per dose) on the acute (Phase I; A,B) and inflammatory (Phase II; C–D) phase in male (A,C) and female (B,D) mice compared to vehicle (VEH; n=9–15) in wild-type (WT) (lined bars) and S426A/S430A mutant (solid bars) mice. Bars represent mean +/− SEM. Asterisk(s) indicate significant
Phase II (Inflammatory phase)
Two-way ANOVA revealed main effects of dose for male (F(3,42)=12.87; p<0.001) and female (F(3,42)=18.03; p<0.001) mice. Female [(F(1,42)=5.04; p=0.030)] but not male (p=0.797) S426A/S430A mice display increased sensitivity to CP55,940 compared to sex-matched wild-type controls (Figure 2C–D). Separate two-way ANOVAs at each CP55,940 dose revealed that female mice were less sensitive to the effects of 0.1 mg/kg CP55,940 (F(1,15)=8.57; p=0.010).
Δ9-THC Tolerance
Phase I (Acute phase)
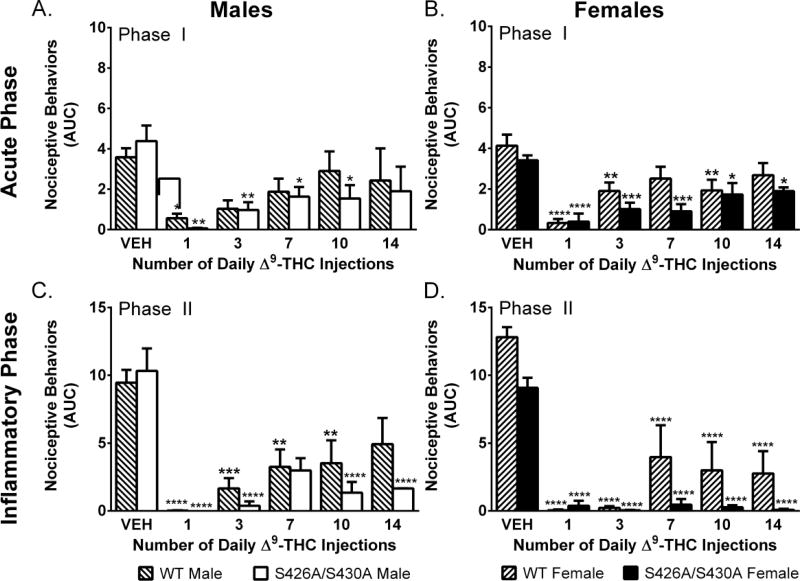
Two-way ANOVA revealed main effects of time (number of Δ9-THC injections) for both male (F(5,64)=4.42; p=0.001) and female (F(5,58)=11.86; p<0.001) mice. Female (F(1,58)=6.79, p=0.012) but not male (p=0.517) S426A/S430A mutant mice showed reduced tolerance to the effects of 6 mg/kg Δ9-THC (Fig 3A–B). Separate ANOVAs comparing each group (sex and genotype) to their vehicle control revealed that male wild-type mice were fully tolerant by day 3; while it took 14 days for male mutants to become tolerant to 6 mg/kg Δ9-THC. Female wild-types were tolerant to Δ9-THC by day 7 whereas mutants failed to develop full tolerance by day 14.
Figure 3. Tolerance to Δ9-THC in the formalin test.
Effects of once daily repeated injections of 6 mg/kg Δ9-THC (n=4–5 per time point) in the acute (Phase I; A–B) and inflammatory (Phase II; C–D) phase of the formalin test in male (A,C) and female (B,D) mice compared to vehicle (VEH) (n=9–15) in wild-type (WT) (lined bars) and S426A/S430A mutant (open bars) mice. Bars represent mean +/− SEM. Asterisk(s) indicate significant differences from vehicle; * p<0.05, **p< 0.01, ***p<0.001, ****p<0.0001.
Phase II (Inflammatory phase)
Two-way ANOVAs revealed main effects of time (number of Δ9-THC injections) for both male (F(5,64)=11.13; p<0.001) and female (F(5,58)=30.32; p<0.001) mice and a main effect of genotype among female (F(1,58)=11.77; p=0.001) but not male (p=0.232) mice. As in the acute phase, female mutants showed reduced tolerance to the effects of 6 mg/kg Δ9-THC (Figure 3C–D). Separate ANOVAs comparing mice to their vehicle control found that only wild-type males were fully tolerant to 6 mg/kg Δ9-THC by day 14.
CP55,940 Tolerance
Phase I (Acute phase)
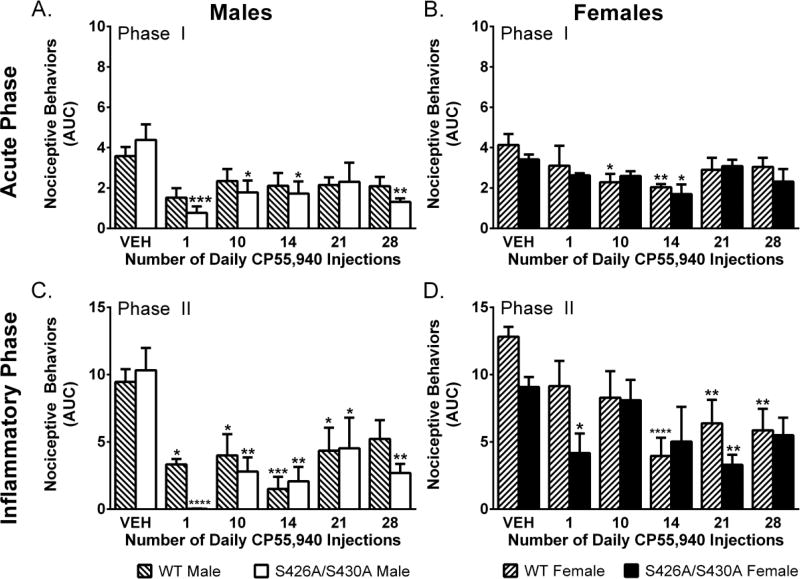
Two-way ANOVAs showed a main effect of time (number of CP55,940 injections) for both male (F(5,63)=3.52; p=0.007) and female (F(5,60)=3.28; p=0.011) mice for the effects of 0.1 mg/kg CP55,940. However, there was no effect of genotype in male (p=0.548) or female (p=0.303) mice (Figure 4A–B). Wild-type and mutant females displayed increased CP55,940 sensitivity over time as differences from vehicle emerged on day 10 for mutant mice only and day 14 for both mutant and wild-types (Figure 4A–B).
Figure 4. Tolerance to CP55,940 in the formalin test.
Effects of once daily repeated injection of 0.1 mg/kg CP55,940 (n=4–5 per time point) in the acute (Phase I; A–B) and inflammatory (Phase II: C–D) phase of the formalin test in male (A,C) and female (B,D) mice compared to vehicle (VEH) (n=9–15) in wild-type (WT) (lined bars) and S426A/S430A mutant (open bars) mice. Bars represent mean +/− SEM. Asterisk(s) indicate significant differences from vehicle; * p<0.05, **p< 0.01, ***p<0.001, ****p<0.0001.
Phase II (Inflammatory phase)
Two-way ANOVA revealed a main effect of time (number of CP55,940 injections) for both male (F(5,63)=7.07; p<0.001) and female (F(5,60)=5.68; p<0.001) mice. There was a main effect of genotype among female (F(1,60)=5.10; p=0.028) but not male (p=0.334) mice. Separate ANOVAS found that male wild-type but not mutant mice were fully tolerant by day 28. As in the acute phase, wild-type and mutant female mice appeared to become more sensitive to the effects of CP55,940 over time as differences from vehicle emerged at days 14, 21 and 28 for wild-type and at day 21 for mutant mice (Figure 4C–D).
Discussion
Sex and genotype differences in sensitivity to Δ9-THC and CP55,940 were evident in both phases of the formalin test. Generally, male mice were more sensitive to the effects of both Δ9-THC and CP55,940 while S426A/S430A mutant mice of both sexes displayed increased sensitivity to these cannabinoid agonists. Cannabinoid tolerance for these agonists also appears to be sex and genotype-dependent. Female mice show delayed tolerance to Δ9-THC relative to male mice while S426A/S430A mutant mice of both sexes show slower tolerance to Δ9-THC. Female but not male mice appear to become more sensitive to the effects of CP55,940 over time. The current study largely confirms our previous work showing that the S426A/S430A mutation attenuates tolerance to the antinociceptive effects of Δ9-THC in males using the tail-flick test to measure acute thermal nociception [9]. However, this study expands that previous work by demonstrating that cannabinoid tolerance is altered by the S426A/S430A mutation in a model of pathological inflammatory pain.
The general finding that males were more sensitive to the effects of both Δ9-THC and CP55,940 and developed tolerance more rapidly to Δ9-THC contrasts with previous work examining cannabinoid response in rats. These previous studies demonstrated that female rats are more sensitive to the antinociceptive effects of Δ9-THC and develop tolerance more rapidly than male counterparts [14,20]. However, it is important to note that this is the first study to examine cannabinoid response and tolerance in mice in the formalin model. The difference between our current work with mice and previous studies with rats raises the possibility of species-dependent sex differences in cannabinoid response and tolerance. In addition, our current study examines sex differences using a model of pathological inflammatory pain while previous studies examining cannabinoid response and tolerance in rats used models of acute thermal nociception [14].
It has been hypothesized that differences in cannabinoid tolerance as a function of sex may be due to differences in acute sensitivity such that the sex with greater acute sensitivity will show a more rapid tolerance development [14]. This hypothesis is consistent with our current findings that male mice are more sensitive to the effects of Δ9-THC causing them to develop tolerance to Δ9-THC more quickly than females. It has been proposed that giving different but equally efficacious doses to males and females could remove this difference in sensitivity and allow for the role of sex on the development of tolerance alone to be examined [14]. Sex differences in the pharmacokinetics of cannabinoid metabolism is another possibility that is not mutually exclusive, and could explain species-dependent sex differences in cannabinoid response. Additional work should examine the possibility that cannabinoid metabolism might be more rapid in female mice versus male mice while the reverse might be the case in rats.
This study is the first to show increased sensitivity of male mice to Δ9-THC and CP55,940 compared to female mice as well as a resistance to tolerance in female mice to Δ9-THC in a model of inflammatory pain. Our findings highlight the importance of including sex as a biological variable in pre-clinical studies involving cannabinoid sensitivity and tolerance. A better understanding of how sex differences influence pain and mediate tolerance to cannabinoids may improve future management of chronic pain in a clinical setting.
Acknowledgments
Funding source: Grants DA036385 (DM), DA037355 (DM) and Department of Comparative Medicine endowment funds.
Footnotes
Author disclosure statement: None declared.
Data presented at the Annual International Cannabinoid Research Society (ICRS) Symposium, Montreal, QC, June 22–27, 2017
References
- 1.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769–780. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18(12):1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, McLellan AT. Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. N Engl J Med. 2016;374(13):1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 4.Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006;45(1):50–52. doi: 10.1093/rheumatology/kei183. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage. 2013;46(2):207–218. doi: 10.1016/j.jpainsymman.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 6.D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33(10):2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorelick DA, Goodwin RS, Schwilke E, Schwope DM, Darwin WD, Kelly DL, et al. Tolerance to effects of high-dose oral δ9-tetrahydrocannabinol and plasma cannabinoid concentrations in male daily cannabis smokers. J Anal Toxicol. 2013;37(1):11–16. doi: 10.1093/jat/bks081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RT, Benowitz NL, Herning RI. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol. 1981;21(8–9 Suppl):143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 9.Morgan DJ, Davis BJ, Kearn CS, Marcus D, Cook AJ, Wager-Miller J, et al. Mutation of putative GRK phosphorylation sites in the cannabinoid receptor 1 (CB1R) confers resistance to cannabinoid tolerance and hypersensitivity to cannabinoids in mice. J Neurosci. 2014;34(15):5152–5163. doi: 10.1523/JNEUROSCI.3445-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen PT, Schmid CL, Raehal KM, Selley DE, Bohn LM, Sim-Selley LJ. β-arrestin2 regulates cannabinoid CB1 receptor signaling and adaptation in a central nervous system region-dependent manner. Biol Psychiatry. 2012;71(8):714–724. doi: 10.1016/j.biopsych.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley JL, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74(2–3):181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 12.Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103(1):156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014;136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakley AA, Wiley JL, Craft RM. Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 2014;143:22–28. doi: 10.1016/j.drugalcdep.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiley JL. Sex-dependent effects of delta 9-tetrahydrocannabinol on locomotor activity in mice. Neurosci Lett. 2003;352(2):77–80. doi: 10.1016/j.neulet.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann ES, Weerts EM, Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp Clin Psychopharmacol. 2015;23(6):415–421. doi: 10.1037/pha0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan SS, Secades-Villa R, Okuda M, Wang S, Pérez-Fuentes G, Kerridge BT, et al. Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug Alcohol Depend. 2013;130(1–3):101–108. doi: 10.1016/j.drugalcdep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson GS, Sufka KJ, Coderre TJ. Optimal scoring strategies and weights for the formalin test in rats. Pain. 1997;70(1):53–58. doi: 10.1016/s0304-3959(96)03299-x. [DOI] [PubMed] [Google Scholar]
- 20.Wakley AA, Craft RM. Antinociception and sedation following intracerebroventricular administration of Δ9-tetrahydrocannabinol in female vs. male rats. Behav Brain Res. 2011;216(1):200–206. doi: 10.1016/j.bbr.2010.07.037. [DOI] [PubMed] [Google Scholar]