Fig. 6.
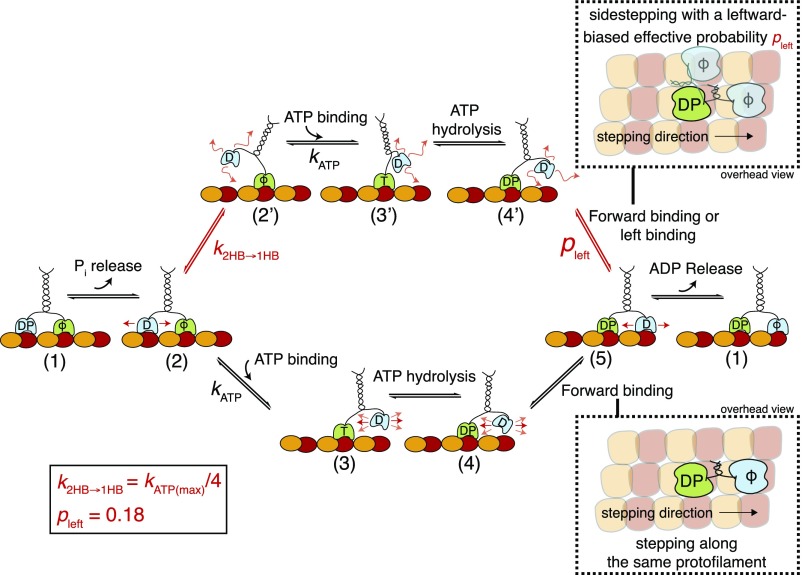
Model of the Kip3 sidestepping mechanism. After completing a step, the motor has released ADP from the leading head and has both heads tightly bound to the microtubule (1). After Pi release from the trailing head, the interaction of this head with the microtubule becomes weaker, but both heads are still bound to the microtubule (2). While waiting for ATP in this 2HB conformation, a bifurcation in the pathway occurs: The leading head (lower path) binds ATP () with the motor in the 2HB conformation or the trailing head (upper path) unbinds from the microtubule before ATP binding and the motor transitions to a 1HB conformation (2′). This transition () is nucleotide independent and can explain different sidestepping probabilities per forward step: If the leading head (lower path) binds ATP with the motor in the 2HB conformation (2), the trailing head moves forward (3), and ATP hydrolysis () induces the binding of this head (4 → 5). Here, the neck linker is restricted and binding is limited to the tubulin dimer directly in front of the bound head (forward stepping on the same protofilament). If the leading head (upper path) binds ATP with the motor in the 1HB conformation (2′), the trailing head is moved forward (3′), and ATP hydrolysis also induces binding (4′ → 5). However, here the neck linker is less restricted and the unbound head can access more binding sites (e.g., forward and leftward). If we assume that the most probable binding site alternative to the one in the front is directly to the left of the bound head (21), we can add a probability pleft (= 0.18) for switching to the protofilament on the left. At physiological ATP conditions (1 mM), both pathways are possible but the effective sidestepping probability is limited because the transition from the 2HB to the 1HB conformation is less likely than ATP binding to the motor in the 2HB conformation (). In contrast, at low ATP conditions, the 1HB conformation becomes more likely, leading to an increased sidestepping probability. Consequently, the effective sidestepping probability saturates (approaching ) at limiting ATP concentration where the 1HB conformation (2′) is predominantly occupied. Here, T denotes ATP, D denotes ADP, DP denotes ADP plus phosphate, and ϕ denotes no nucleotide. Loose binding and diffusion are represented by small red arrows.